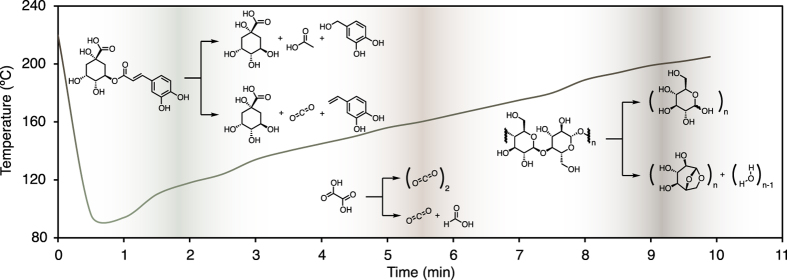
Figure 1. The roast profile for the Tanzanian Burka (Has Bean).
In this case, 10 kg of the Burka coffee was roasted in a 12 kg Probat Roaster. The temperature was monitored with a probe in the headspace of the oven, and hence the hot air rapidly cools due to thermal energy transfer to the green coffee. The temperature trajectory throughout the roasting process determines the decomposition of organic materials in coffee. Three illustrative decomposition reactions are shown that are representative processes throughout the heating process. At lower temperature a chlorogenic acid (left) may decompose through either hydrolysis or pyrolysis into quinic acid, acetic acid and the phenolic compound 3,4-dihydroxybenzyl alcohol40, or quinic acid, carbon dioxide and 3,4-dihydroxystyrene41,42. Oxalic acid (centre) may decarboxylate to either CO2 or in the case of incomplete combustion CO2 and formic acid19. At higher temperatures cellulose can undergo hydrolysis to smaller sugar derivatives including glucose and levoclucosan43,44,45. Both the temperature and time determine the chemical composition of the roasted coffee: In this case, the coffee was removed from the oven after 9 m 54 s as this time was determined to result in a soluble, sweet and favourably acidic product.