Graphical abstract
A water-soluble 1,1-geminal fluorinated bisphosphonate showing a single and narrow 19F resonance was synthesised and characterised. Its properties as contrast agent for 19F-MRI were evaluated in vitro and in vivo.
Keywords: 19F-MRI, Bisphosphonates (BPs), Magnetic resonance imaging (MRI), Preclinical imaging, Fluorinated bisphosphonate
Highlights
-
•
A trifluoromethyl-bisphosphonate for 19F-MRI was synthesised and characterised.
-
•
In vitro studies showed its potential with properties comparable to previous probes.
-
•
In vivo 19F/1H-MRI studies showed fast renal excretion and liver uptake.
Abstract
19F-magnetic resonance imaging (MRI) is a promising technique that may allow us to measure the concentration of exogenous fluorinated imaging probes quantitatively in vivo. Here, we describe the synthesis and characterisation of a novel geminal bisphosphonate (19F-BP) that contains chemically-equivalent fluorine atoms that show a single and narrow 19F resonance and a bisphosphonate group that may be used for labelling inorganic materials based in calcium phosphates and metal oxides. The potential of 19F-BP to provide contrast was analysed in vitro and in vivo using 19F-MRI. In vitro studies demonstrated the potential of 19F-BP as an MRI contrast agent in the millimolar concentration range with signal-to-noise ratios (SNR) comparable to previously reported fluorinated probes. The preliminary in vivo MRI study reported here allowed us to visualise the biodistribution of 19F-BP, showing uptake in the liver and in the bladder/urinary system areas. However, bone uptake was not observed. In addition, 19F-BP showed undesirable toxicity effects in mice that prevent further studies with this compound at the required concentrations for MRI contrast. This study highlights the importance of developing 19F MRI probes with the highest signal intensity achievable.
1. Introduction
MRI is a medical imaging technique that offers high-resolution images of soft tissues without the need for ionising radiation. In addition, and unlike other techniques such as those based on radionuclides, it does not require the injection of contrast agents in order to obtain meaningful images. However, for some imaging procedures such as angiography or molecular imaging, chemical compounds can be used to enhance the contrast of the specific tissue of interest. In this context, one area that MRI currently lags behind other imaging modalities, particularly positron emission tomography (PET) and single photon emission computed tomography (SPECT), is the quantitative measurement of the signal provided by these contrast agents. This is a key requirement for molecular imaging applications. Current contrast-based MR techniques rely on the detection of imaging agents containing paramagnetic ions such as gadolinium, manganese or iron. However, interpretation of the results is difficult due to the varying underlying signal hyper- and hypo-intensities in MRI. In answer to this 19F-MRI has been implemented. The use of fluorine as the nucleus for magnetic resonance has several advantages over protons. First, the lack of endogenous MR-visible fluorine provides an unambiguous readout of the introduced fluorine-containing compounds location. In addition the 19F MR signal can be quantified, giving a measure of the contrast agent’s concentration. This is in contrast to paramagnetic contrast agents used in 1H-MRI and based on Gd, Mn and particularly Fe, where in vivo absolute quantification is not achievable.
The main uses of 19F-MRI in biomedical imaging to date has been for cell tracking [1], [2], [3], [4], [5] visualisation of inflammation [6], [7], [8], [9] and for imaging angiogenesis [10], [11] all using 19F nanoparticles. This is an obvious choice due to the capacity of nanoparticles to carry the many fluorine atoms required to obtain sufficient signal. More recently attempts have been made to image smaller compounds by modulating the 19F signal using lanthanide metals [12], [13] and used for the detection of gene expression [14]. Despite these early promising results and clear advantages for molecular imaging compared to 1H-MRI, 19F-MRI remains underused in clinical practice. This is due to a major disadvantage, which is low sensitivity [15]. As a consequence most 19F-MRI probes designed to date need to have many fluorine atoms to provide enough signal in the tissues of interest (∼20–50 mM 19F). However, the number of fluorine atoms that a molecule can carry is limited for several reasons. First is solubility, as the fluorine content of a molecule increases, the water solubility decreases. The second limitation is the number of 19F signals, the ideal 19F-MRI contrast agent having one single narrow resonance to maximise signal and avoid imaging artifacts. To achieve this all the fluorine atoms must be in the same chemical and magnetic environment. Another limitation of 19F-MRI is related to the long longitudinal relaxation times (T1) of the fluorine nucleus (∼1–2 s). This translates into long acquisition times for the MRI procedure due to the 5–10 s required between radiofrequency (RF) pulses, which results in long times or more complex non-standard MRI sequences.
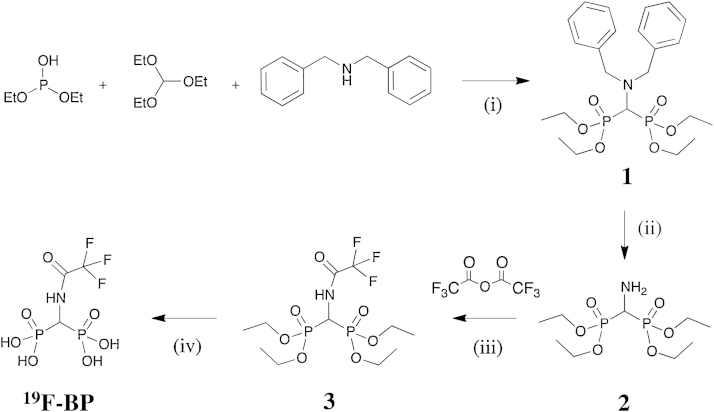
We are interested in developing 19F-MRI contrast agents for molecular imaging that show single and narrow 19F resonances and short T1 relaxation times. Previously we have shown that 1,1-bisphosphonates (BPs) bind very strongly to metabolically active bone and calcium phosphate materials such as hydroxyapatite using SPECT and PET imaging [16], [17], [18], [19]. In addition, we found that BPs also bind very strongly to many nanomaterials based on lanthanide metal oxides of the type M2O3 (M = Gd, Er, among others) with known relaxation rate-enhancement properties [19]. We hypothesised that a fluorinated BP molecule could be an useful tool in the development of 19F-MRI probes, that would allow to combine of the amplification properties of nanoparticle-based platforms (high numbers of equivalent fluorine atoms) with the relaxation-enhancement properties of lanthanide-based materials (short acquisition times) without affecting their water solubility. In this way we could potentially achieve 19F-MRI probes with high signal intensity and sensitivity that could be imaged in a short time. In addition, their solution and in vivo properties could be easily controlled by surface modification using the same BP chemistry. In this work, we report our first attempts at achieving this aim by synthesizing and characterising a new fluorinated BP (19F-BP, Scheme 1) and evaluate for the first time its properties as a single molecule for 19F-MRI in vitro and in vivo.
Scheme 1.
The synthetic scheme of 19F-BP. (i) 29 h at 150–160 °C; (ii) H2, 10% Pd/C catalyst in EtOH, room temperature; (iii) 3 h in dry DCM; (iv) (a) 24 h, Me3SiBr (15 eq) in dry DCM, room temperature (b) 1.5 h MeOH, 1.5 mL, room temperature.
2. Results and discussion
2.1. Synthesis
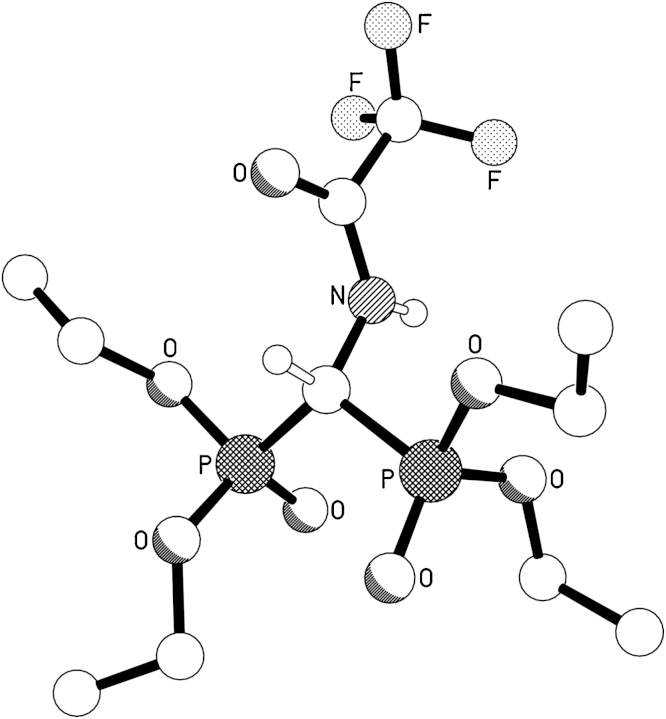
The reaction scheme for the synthesis of 19F-BP is shown in Scheme 1. Tetraethyl aminomethyl-bisphosphonate (2) was synthesized following published methods [20], [21]. Briefly, diethyl phosphite, triethylorthoformate and dibenzylamine were reacted for 29 h at 150–160 °C to yield the benzylated bisphosphonate (1). The amino group of 1 was deprotected with H2 and 10% Pd/C catalyst to yield 2. After removal of the catalyst, 2 was reacted with 2.9 equivalents of trifluoroacetic anhydride (TFAA) in dry DCM for 3 h. Excess TFAA was used in order to prevent low reaction yields due to potential hydrolysis of the anhydride. After evaporation of the volatiles and work-up, 3 was recrystallised from cold hexanes in good yields (78%). The compound was characterised by NMR, HR-MS and the structure confirmed by X-ray crystallography (Fig. 1 and Fig. SI)
Fig. 1.
The molecular structure of 3 (CCDC ID 960161).
The ethyl-protected bisphosphonate group of 3 was deprotected by reacting with excess bromotrimethylsilane followed by methanolisis at room temperature. The reaction gave quantitative yields of 19F-BP as assessed by NMR and MS, confirming complete removal of the ethyl protecting groups. 19F-NMR and 31P-NMR also confirmed the stability of the trifluoromethyl and bisphosphonic groups, respectively. The solubility properties of 3 changed from hydrophobic to hydrophilic after deprotection, as expected for bisphosphonic acids, and allowed us to perform our imaging studies in water. One of the main advantages of this compound over most 19F-MRI contrast agents reported to date based on perfluorinated molecules is the chemical equivalence of its F atoms. Non-equivalent F atoms result in broad and/or multiple resonances that have a negative effect on the final 19F-MRI signal. In 19F-BP, however, having a narrow single 19F resonance (−76.15 ppm, ω1/2 = 4.9 Hz), maximises imaging signal and minimises the appearance of image artefacts.
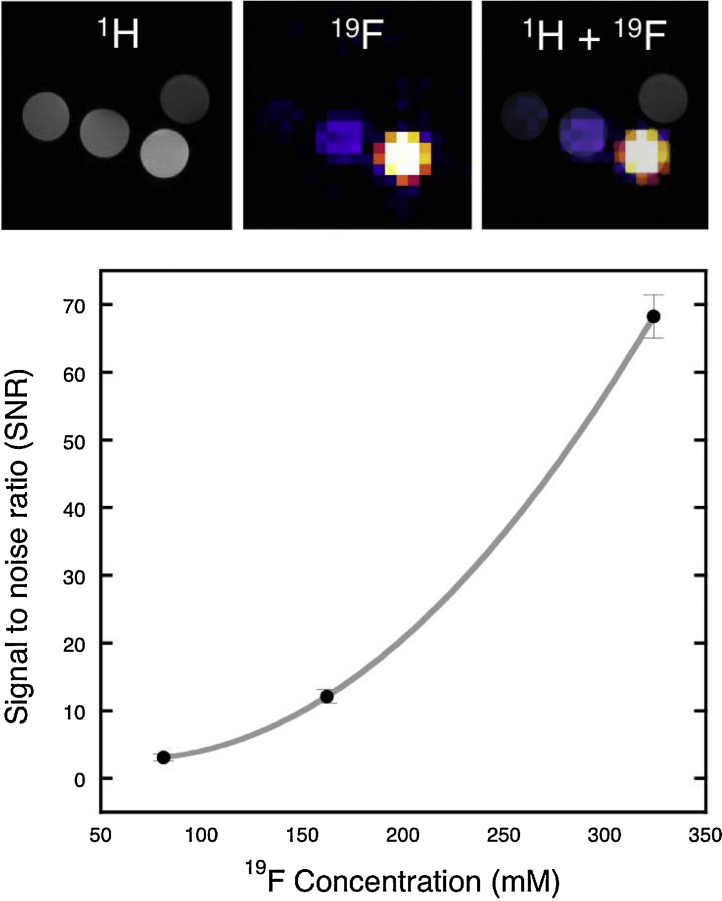
2.2. In vitro MR imaging studies
Phantom MRI studies were performed to evaluate the contrast properties of 19F-BP (Fig. 2). The compound was dissolved in water at pH 7 at several concentrations (27, 54 and 108 mM) and imaged in a preclinical 9.4 T MRI scanner. A clear concentration-dependent increase in signal intensity and signal to noise ratio (SNR) was found, demonstrating that 19F-BP can be imaged in the high mM concentration range. Stability studies were also performed using these samples. The 1H NMR and 19F-MRI spectra remained stable for 5 h at pH 7 and 37 °C, confirming the stability of 19F-BP at these conditions. This gave us confidence to study its biodistribution properties in vivo.
Fig. 2.
In vitro MR imaging study. Top: 1H and 19F MRI phantom study with vials of 19F-BP at increasing concentrations (27, 54 and 108 mM) in water from left to right, with water above. Bottom: graph showing the increase of signal to noise ratio (SNR) with increasing probe concentration. The fit is a smooth curve fit intended to represent the trend. Error bars are the result of 3 ROI image analyses.
2.3. In vivo MR imaging studies
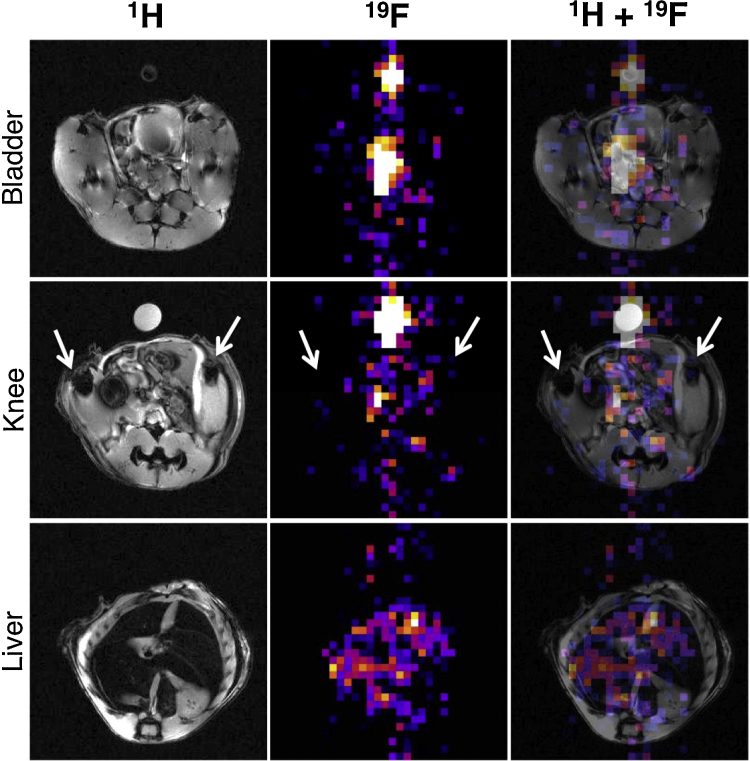
Preliminary in vivo studies were carried out in a 9.4 T scanner with a healthy mouse. We have recently shown that bifunctional BPs accumulate in areas of high bone metabolism such as the end of long bones and bone metastases using SPECT imaging [17], [18]. Hence, we expected 19F-BP to accumulate in bone. However, after intravenous injection, only signals in the bladder/urinary system and liver areas were detected, the former most probably due to renal excretion as expected for a molecule of this size (Fig. 3A) although this cannot be confirmed with the data available. In addition, uptake in other tissues/organs of the same area such as the uterus cannot be ruled out. It is important to note that the 19F and 1H acquisitions were not performed simultaneously and each modality was acquired with different slice thicknesses (19F is 5 times thicker than the 1H image), complicating the interpretation of the images. Motion artifacts could also be responsible for the suboptimal overlay of the two modalities. The signal observed in the liver area (Fig. 3C), which is a much bigger organ and hence less affected by these issues (Fig. 3C), is more conclusive to uptake by this organ. Liver uptake is common for lipophilic molecules, and since fluorination is known to increase the lipophilicity of compounds, it is likely to be the result of the trifluoromethyl group. We believe that the lack of bone uptake may be the result of its high lipophilicity, compared to non-fluorinated BPs, resulting in higher liver uptake, and/or fast renal clearance. Indeed, recent reports support the notion that fluorinated groups increase the renal excretion of molecules in vivo [22]. Another interesting possibility is that bone binding could have resulted in a chemical shift of the 19F resonance that could result in a lack of signal from bone. However, the presence of the expected single resonance in the broad sweep width spectrum performed prior to the imaging session strongly suggests this is not the case.
Fig. 3.
Animal MR imaging study (see Section 4 for details). A mouse was injected with 19F-BP i.v. (108 mM in PBS buffer) and imaged using 1H (left column) and 19F MRI (middle column), which were overlayed to determine location (right column). Top row is an axial slice through the bladder/urinary tract area, second row an axial slice through the knees (arrows) and the third row an axial slice through the liver. A vial containing a known amount of 19F-BP was positioned next to the animal for reference.
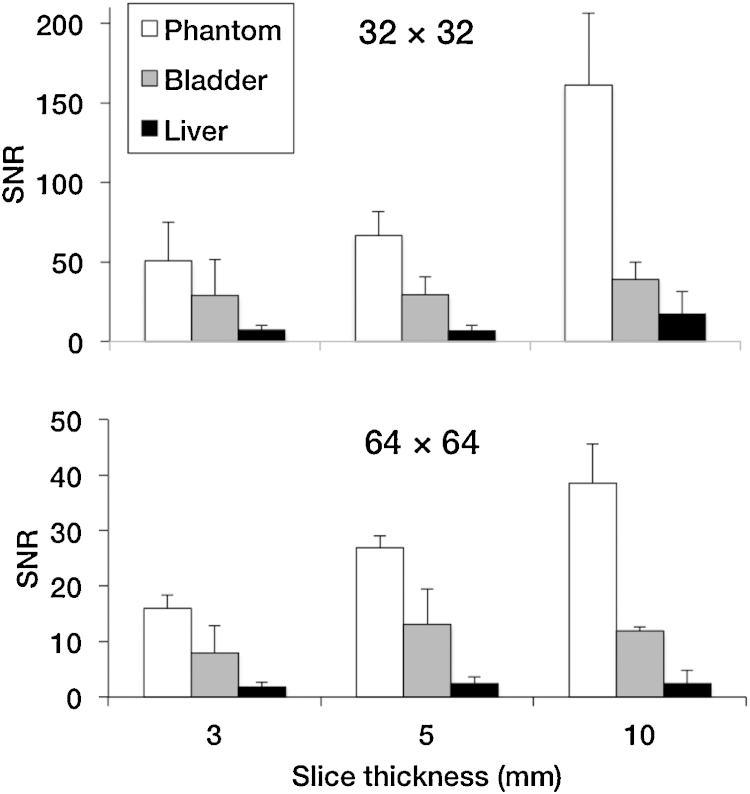
Another potential reason for the lack of bone uptake observed could be a low signal to noise ratio (SNR). SNR measurements are important in 19F-MRI and provide a measure of sensitivity (i.e. contrast achieved with amount of imaging agent injected). SNR values of a phantom sample with 19F-BP were found to be in the 50–150 range (32 × 32 matrix size) and 15–40 range (64 × 64 matrix size) for different slice thicknesses. The size of the matrix size is indirectly proportional to the sensitivity, hence the higher values obtained at 32 × 32. For the mouse studies these values were found to be in the 10–40 and 2–12 range, and compare favourably to other animal studies from Bible et al. [23] and Giraudeau et al. [24] (Fig. 4). It is important to note, however, that 19F-BP was found to be toxic at concentrations required to achieve in vivo MRI signal (97–119 mM). While other BPs used for nuclear imaging such as 99mTc-MDP are required in micromolar concentrations to obtain image contrast, the amount of BPs required for MRI contrast or therapy is much higher. Toxicity has been observed in animal studies with an amino-bisphosphonate used for therapeutic purposes and injected intravenously (alendronate), at doses of 20 mg/kg. However, doses of 150 mg/kg are required for detecting the 19F-MRI signal of 19F-BP (for a 20 g mouse). Hence, toxicity is likely to be the result of the bisphosphonate and not the trifluoromethyl group, although further studies are required to confirm this. These results prompted us to abandon the study of 19F-BP for bone imaging and look for potential strategies in order to increase its sensitivity.
Fig. 4.
Phantom and animal MR imaging study SNR values. SNR values were calculated for a variety of imaging parameters: matrix size (32 × 32, top; 64 × 64, bottom) and slice thickness (x-axis), using 19F MRI in the phantom, bladder and liver. Error bars are the result of 3 ROI image analyses.
2.4. Potential strategies to improve sensitivity
The most obvious strategy to improve the sensitivity of 19F-BP is to increase the number of F atoms in the molecule. Interestingly, there are some recent synthetic strategies that would allow us to synthesise a similar BP with several chemically-equivalent F atoms [22]. However, an increase in fluorine content will likely have two main adverse effects. First is solubility, as we anticipate the water solubility will decrease and eventually may result in water-insoluble compounds. The second effect is related to this lower hydrophilicity. We have observed a high degree of liver uptake and hence lipophilicity with a trifluoromethyl group, addition of more fluorine atoms will probably worsen this effect. Another potential adverse effect would be the observed increased in vivo rate of excretion of fluorinated agents others and we have observed [22]. A recent proposed method to improve the sensitivity of 19F-MRI contrast agents is by positioning the F atoms near a lanthanide in order to enhance their relaxation rates. This technique has been recently explored by Parker and Blamire et al. showing this strategy can result in lower acquisition times and detection limits by as much as 2 orders of magnitude [12], [13], [25]. We hypothesised that, given the known ability of BPs to chelate Ln3+ metals and lanthanide oxide materials [19], we could explore this property to enhance the relaxation rate of 19F-BP and hence increase its sensitivity. This method, of course, would not be useful for bone imaging, unless other bifunctional BPs that contain a Ln3+ binding group such as a macrocycle chelate (leaving the BP free to bind to bone mineral or inorganic material) between the F-containing motif and the BP are designed. However, it could provide a very useful method to label lanthanide-containing nanomaterials with large numbers of 19F atoms and fast acquisition times for other purposes such as cell tracking or molecular imaging using 19F-MRI. A preliminary in vitro MR study in which we measured the longitudinal and transverse relaxation rates (R1 and R2) of 19F-BP in the absence and presence of 1 molar equivalent of different lanthanide salts (Dy3+, Er3+, Gd3+, Ho3+ and Tb3+) supports the potential of this approach as the presence of Ln3+ metals in the solution enhance both relaxation rates by as much as 3 orders of magnitude (supporting information). It is important to note, however, that well-defined and characterised 19F-BP-Ln3+ complexes would be required in order to validate these findings. We believe this is a strategy that would be particularly useful in conjunction with nanoparticle systems that can combine large numbers of —CF3 groups with lanthanide metals at the surface and the required distance from each other. Using this combination, high sensitivity (signal/mole contrast agent) may be achieved thanks to the high numbers of chemically-equivalent 19F atoms (hundreds to thousands for a spherical nanometer size particle) with the relaxation capabilities of paramagnetic metals. In addition to the use of paramagnetic ion relaxation, the sensitivity could be further increased in the future by using more efficient MR protocols such as ultrafast sequences recently developed [26].
3. Conclusions
19F-BP was successfully synthesised and characterised. The compound is water soluble and stable and shows a single and narrow fluorine resonance ideally suited for 19F-MRI. Phantom studies show that 19F-BP can be imaged using a 9.4 T magnet in the high mM range with SNR ratios similar to other reported probes. An in vivo 19F-MRI study strongly suggests that 19F-BP was rapidly excreted renally although uptake by other organs/tissue in the area cannot be completely ruled out with our data. Uptake in the liver was also observed which is probably a result of the lipophilicity of the trifluoromethyl group. This data suggests that the lack of bone uptake observed, the natural target of BPs, may be due to the presence of the fluorinated group resulting in fast clearance, as other studies have recently found [22]. More importantly, 19F-BP was found to be toxic at the concentrations used in this study. From these results it is clear that, while 19F-BP may not be useful for bone imaging by itself it may be an useful compound to provide 19F signal to many inorganic materials of known affinity towards BPs such as calcium phosphates (i.e. hydroxyapatite) and metal oxides, as our recent work suggests. Future work is aimed at using 19F-BP and related BPs to fully exploit this approach.
4. Experimental
4.1. Materials
Reagents and starting materials were obtained from commercial sources and used as received unless otherwise noted. Organic solvents were of HPLC grade. Water (Type I, 18.2 MΩ cm) was obtained from an ELGA Purelab Option-Q system. Dittmer-Lester’s TLC reagent for the detection of phosphorus was prepared following the original literature protocol [27]. NMR spectra were obtained in a 400 MHz Bruker Avance III (Germany). 1H chemical shifts are referenced with respect to the residual solvent peak (δH 4.79 ppm, D2O; 7.26 ppm CDCl3) [28]. 31P resonances were referenced to an external solution of 85% H3PO4 (δP 0 ppm). 13C chemical shifts were referenced to the residual solvent peak (δC 77.16 ppm, CDCl3) or left unreferenced (D2O). 19F resonances were referenced to an external solution of TFA (δF −78.5 ppm). High-resolution mass spectra (HR-MS) were obtained using an Agilent 6500 Accurate-Mass Q-TOF LC–MS system using electrospray ionization. Tetraethyl((dibenzylamino)methylene)bisphosphonate (1) and tetraethyl(aminomethylene)bisphosphonate (2) were synthesised following published methods [20], [21].
4.2. Syntheses
4.2.1. Tetraethyl((2,2,2-trifluoroacetamido)methylene)bisphosphonate. 3
2 (200 mg, 0.66 mmol) was dissolved in dry drychloromethane (200 cm3) under nitrogen and the flask cooled to 0 °C. After 5 min, trifluoroacetic anhydride (0.274 cm3, 1.9 mmol) was added in small portions over 2 min. The ice bath was then removed and the solution was left stirring at room temperature for 2 h during which time the reaction mixture turned slightly yellow. The volatiles were then removed under reduced pressure leaving a clear yellow residue. This residue was dissolved in 2 cm3 of dichloromethane and to this mixture were added increasing amounts of a 1% solution of sodium bicarbonate followed by shaking, until the pH of the aqueous layer was 7 (∼10 cm3). The organic layer was separated and washed with 3 cm3 of water, dried over sodium sulfate, filtered and evaporated under reduced pressure. The residue recrystallised from hexanes after 24 h standing at 4 °C, yielding large quantities of X-ray diffraction-quality crystals (78% yield).
1H NMR (CDCl3, 400.3 MHz, 298 K) δH (ppm) 4.16 (m, 8H, (—P(O)(OCH2CH3)2)), 3.56 (t, 2JH-P = 22 Hz, 1H, ((P(O)(OEt)2)2—CH—NH—)), 1.34 (s, 12H, (P(O)(OCH2CH3)2)); 13C NMR (CDCl3, 100.7 MHz, 298 K) δC (ppm) 159.1 (q, 2JC-F = 40 Hz, (—NH—CO—CF3)), 115.6 (q, JC-F = 291 Hz, (—NH—CO—CF3)), 65.3 (t, 2JC-P = 3 Hz, (—P(O)(OCH2CH3)2)), 43.9 (t, JC-P = 148 Hz, ((P(OEt)3)2—CH—NH—)), 16.0 (bs, (—P(O)(OCH2CH3)2)); 31P{1H}-NMR (161.9 MHz, CDCl3, 298 K) δP (ppm) 14.02; 19F-NMR (376 MHz, CDCl3, 298 K) δF (ppm) −76.47; HR-MS (ESI) 400.0939 (M + H+, found), 400.0932 (M + H+, calculated). 422.0759 (M + Na+, found), 422.0721 (M + Na+, calculated).
4.2.2. ((2,2,2-trifluoroacetamido)methylene)bisphosphonic acid. 19F-BP
3 (89 mg, 0.22 mmol) was dissolved in dry drychloromethane (200 cm3) under nitrogen and the flask cooled to 0 °C. After 5 min, bromotrimethylsilane (0.444 cm3, 3.3 mmol) was added dropwise over 5 min. The ice bath was then removed and the solution was left stirring under nitrogen at room temperature for 24 h during which time the solution turned yellow. The volatiles were then removed under reduced pressure and the residue dissolved in 1.5 cm3 of methanol, resulting in a colourless solution. The reaction was left stirring at room temperature for a further 1.5 h followed by evaporation under reduced pressure yielding the product in quantitative yield as a clear sticky oil.
1H NMR (D2O, 400.3 MHz, 298 K) δH (ppm) 4.60 (t, 2JH-P = 22 Hz, 1H, ((P(O)(OH)2)2—CH—NH—)); 13C NMR (D2O, 100.7 MHz, 298 K) δC (ppm) 158.3 (q, 2JC-F = 40 Hz, (—NH—CO—CF3)), 115.8 (q, JC-F = 285 Hz, (—NH—CO—CF3)), 47.3 (t, JC-P = 136 Hz, ((P(OH)2)2—CH—NH—)); 31P{1H}-NMR (161.9 MHz, D2O, 298 K) δP (ppm) 12.07; 19F-NMR (376 MHz, D2O, 298 K) δF (ppm) −76.15; HR-MS (ESI) 287.9664 (M + H+, found), 287.9650 (M + H+, calculated).
4.3. X-ray crystallography
Crystal data for 3: C11H22F3NO7P2, M = 399.24, triclinic, P-1 (no. 2), a = 10.2420(5), b = 10.2485(5), c = 10.5343(5) Å, α = 65.615(4), β = 71.735(4), γ = 70.761(4)°, V = 930.40(9) Å3, Z = 2, Dc = 1.425 g cm−3, μ(Cu-Kα) = 2.700 mm−1, T = 173 K, colourless blocks, Oxford Diffraction Xcalibur PX Ultra diffractometer; 3670 independent measured reflections (Rint = 0.0228), F2 refinement, 29R1(obs) = 0.0352, wR2(all) = 0.0972, 3257 independent observed absorption-corrected reflections [|Fo| > 4σ(|Fo|), 2θmax = 145°], 249 parameters. Crystallographic data for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre (CCDC ID 960161). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44 1223 336033 or e-mail: deposit@ccdc.cam.ac.uk).
4.4. Relaxation rate measurements
The relaxation times T1 and T2 of 19F in 19F-BP and 19F-BP + Ln3+ mixtures were measured in H2O at pH 7 at 400 MHz on a Bruker Avance (Bruker, Ettlingen, Germany) and converted to the R1 (1/T1) and R2 (1/T2) rates. T1 measurements were performed using an inversion recovery technique with 8 inversion times between 0.001 and 4 s, TR = 7 s and 256 averages. T2 measurements were performed with a spin echo technique with 12 TEs between 0.002 and 0.2 s, TR = 7 s and 8 averages. Analysis was performed using Top Spin software (Bruker, Ettlingen, Germany).
4.5. Magnetic resonance imaging (MRI)
4.5.1. Phantom imaging
19F-BP at different concentrations (27, 54 and 108 mM) in 250 μL PCR tubes were positioned in a 9.4T Bruker Avance vertical bore scanner using a quadrature volume coil (Bruker, Ettlingen, Germany) alongside a PCR tube containing water. For 1H imaging for localisation a RARE sequence was used with TR = 1500 ms, TE = 8.5 ms, NSA = 1, matrix = 256 × 256, FOV = 30 × 30 mm, slc = 1 mm. For the 19F imaging the coil was tuned to the 19F resonance frequency and a spin echo sequence used with a TR = 3000 ms, TE = 7.6 ms, NSA = 100, matrix = 32 × 32, FOV = 30 × 30 mm, slc = 6 mm, total scan time = 2 h 40 min.
4.5.2. Animal imaging
All animal experiments were performed with licences issued in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 (UK). One female Balb/c mice (Charles River, Edinburgh, UK), 8–10 weeks old, was anaesthetised using 5% and maintained with 1–2% isoflurane, and injected with 100 μL compound (108 mM in PBS) via the tail vein before being transferred to the MRI scanner (9.4T Bruker Avance vertical bore scanner using a quadrature volume coil (Bruker, Ettlingen, Germany)). For 1H imaging a FLASH sequence was used with TR = 350 ms, TE = 5.4 ms, FA = 40°, NSA = 5, matrix = 256 × 256, FOV = 30 × 30 mm, slc = 1 mm, 30 slices. For the 19F imaging the coil was tuned to the 19F resonance frequency and a RARE sequence used with a TR = 1500 ms, TE = 8.5 ms, RARE factor = 4, NSA = 200, matrix = 32 × 32, FOV = 30 × 30 mm, slc = 5 mm, 6 slices, total scan time = 30 min. In addition the same sequence was run, but with FOV = 64 × 64, which had a total scan time of an hour.
19F MR images were overlayed on to the 1H MR images using ImageJ software (National Institutes of Health, US). To calculate the signal to noise ratios (SNR) of the phantom, bladder and liver ROIs were drawn around the object and also in the background and then values inputted into the following equation taking into account Edelsteins correction factor: SNR = Intensity ROI/(STDEV noise)/√(2-π/2) [29].
Acknowledgements
This work was funded by The Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC under Grant No. WT 088641/Z/09/Z. KPS and GDK were funded by the King’s College London and UCL Comprehensive Cancer Imaging Centre funded by the CRUK and EPSRC in association with the MRC and DoH (England). GDK was also part funded by the Rosetrees Trust. The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jfluchem.2016.02.008.
X-ray crystallography data and relaxation rate measurements of 19F-BP in the presence and absence of lanthanide metal salts (Table S1).
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Partlow K.C., Chen J., Brant J.A., Neubauer A.M., Meyerrose T.E., Creer M.H., Nolta J.A., Caruthers S.D., Lanza G.M., Wickline S.A. FASEB J. 2007;21:1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- 2.Boehm-Sturm P., Mengler L., Wecker S., Hoehn M., Kallur T. PLoS One. 2011;6:e29040. doi: 10.1371/journal.pone.0029040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonetto F., Srinivas M., Heerschap A., Mailliard R., Ahrens E.T., Figdor C.G., de Vries I.J.M. Int. J. Cancer. 2011;129:365–373. doi: 10.1002/ijc.25672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivas M., Cruz L.J., Bonetto F., Heerschap A., Figdor C.G., de Vries I.J.M. Biomaterials. 2010;31:7070–7077. doi: 10.1016/j.biomaterials.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 5.Srinivas M., Heerschap A., Ahrens E.T., Figdor C.G., de Vries I.J.M. Trends Biotechnol. 2010;28:363–370. doi: 10.1016/j.tibtech.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flögel U., Ding Z., Hardung H., Jander S., Reichmann G., Jacoby C., Schubert R., Schrader J. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flögel U., Su S., Kreideweiß I., Ding Z., Galbarz L., Fu J., Jacoby C., Witzke O., Schrader J. Am. J. Transplant. 2011;11:235–244. doi: 10.1111/j.1600-6143.2010.03372.x. [DOI] [PubMed] [Google Scholar]
- 8.Southworth R., Kaneda M., Chen J., Zhang L., Zhang H., Yang X., Razavi R., Lanza G., Wickline S.A. Nanomed. Nanotechnol. Biol. Med. 2009;5:359–367. doi: 10.1016/j.nano.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim Y.T., Cho M.Y., Kang J.-H., Noh Y.-W., Cho J.-H., Hong K.S., Chung J.W., Chung B.H. Biomaterials. 2010;31:4964–4971. doi: 10.1016/j.biomaterials.2010.02.065. [DOI] [PubMed] [Google Scholar]
- 10.Waters E., Chen J., Allen J., Zhang H., Lanza G., Wickline S., Cardiov J. Magn. Reson. 2008;10:43. doi: 10.1186/1532-429X-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraudeau C., Geffroy F., Mériaux S., Boumezbeur F., Robert P., Port M., Robic C., Bihan D., Lethimonnier F., Valette J. Angiogenesis. 2013;16:171–179. doi: 10.1007/s10456-012-9310-0. [DOI] [PubMed] [Google Scholar]
- 12.Chalmers K.H., Botta M., Parker D. Dalton Trans. 2011;40:904–913. doi: 10.1039/c0dt01232g. [DOI] [PubMed] [Google Scholar]
- 13.Chalmers K.H., De Luca E., Hogg N.H.M., Kenwright A.M., Kuprov I., Parker D., Botta M., Wilson J.I., Blamire A.M. Chem. Eur. J. 2010;16:134–148. doi: 10.1002/chem.200902300. [DOI] [PubMed] [Google Scholar]
- 14.Mizukami S., Matsushita H., Takikawa R., Sugihara F., Shirakawa M., Kikuchi K. Chem. Sci. 2011;2:1151–1155. [Google Scholar]
- 15.Terreno E., Castelli D.D., Viale A., Aime S. Chem. Rev. 2010;110:3019–3042. doi: 10.1021/cr100025t. [DOI] [PubMed] [Google Scholar]
- 16.Sandiford L., Phinikaridou A., Protti A., Meszaros L.K., Cui X., Yan Y., Frodsham G., Williamson P.A., Gaddum N., Botnar R.M., Blower P.J., Green M.A., de Rosales R.T.M. ACS Nano. 2013;7:500–512. doi: 10.1021/nn3046055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Rosales R.T.M., Finucane C., Foster J., Mather S.J., Blower P.J. Bioconjug. Chem. 2010;21:811–815. doi: 10.1021/bc100071k. [DOI] [PubMed] [Google Scholar]
- 18.de Rosales R.T.M., Finucane C., Mather S.J., Blower P.J. Chem. Commun. 2009:4847–4849. doi: 10.1039/b908652h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Rosales R.T.M., Tavaré R., Paul R.L., Jauregui-Osoro M., Protti A., Glaria A., Varma G., Szanda I., Blower P.J. Angew. Chem. Int. Ed. 2011;50:5509–5513. doi: 10.1002/anie.201007894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantoci D., Denike J.K., Wechter W.J. Synth. Commun. 1996;26:2037–2043. [Google Scholar]
- 21.Kubicek V., Rudovsky J., Kotek J., Hermann P., Elst L.V., Muller R.N., Kolar Z.I., Wolterbeek H.T., Peters J.A., Lukes I. J. Am. Chem. Soc. 2005;127:16477–16485. doi: 10.1021/ja054905u. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Z.X., Liu X., Jeong E.K., Yu Y.B. Angew. Chem. Int. Ed. 2009;48:4755–4758. doi: 10.1002/anie.200901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bible E., Dell'Acqua F., Solanky B., Balducci A., Crapo P.M., Badylak S.F., Ahrens E.T., Modo M. Biomaterials. 2012;33:2858–2871. doi: 10.1016/j.biomaterials.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giraudeau C., Djemaï B., Ghaly M.A., Boumezbeur F., Mériaux S., Robert P., Port M., Robic C., Bihan D.L., Lethimonnier F., Valette J. NMR Biomed. 2012;25:654–660. doi: 10.1002/nbm.1781. [DOI] [PubMed] [Google Scholar]
- 25.Chalmers K.H., Kenwright A.M., Parker D., Blamire A.M. Magn. Reson. Med. 2011;66:931–936. doi: 10.1002/mrm.22881. [DOI] [PubMed] [Google Scholar]
- 26.Schmid F., Holtke C., Parker D., Faber C. Magn. Reson. Med. 2013;69:1056–1062. doi: 10.1002/mrm.24341. [DOI] [PubMed] [Google Scholar]
- 27.Ryu E.K., Maccoss M. J. Lipid Res. 1979;20:561–563. [PubMed] [Google Scholar]
- 28.Gottlieb H.E., Kotlyar V., Nudelman A. J. Org. Chem. 1997;62:7512–7515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 29.Tofts P.S. John Wiley & Sons, Ltd.; 2004. The Measurement Process: MR Data Collection and Image Analysis, Quantitative MRI of the Brain; pp. 17–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.