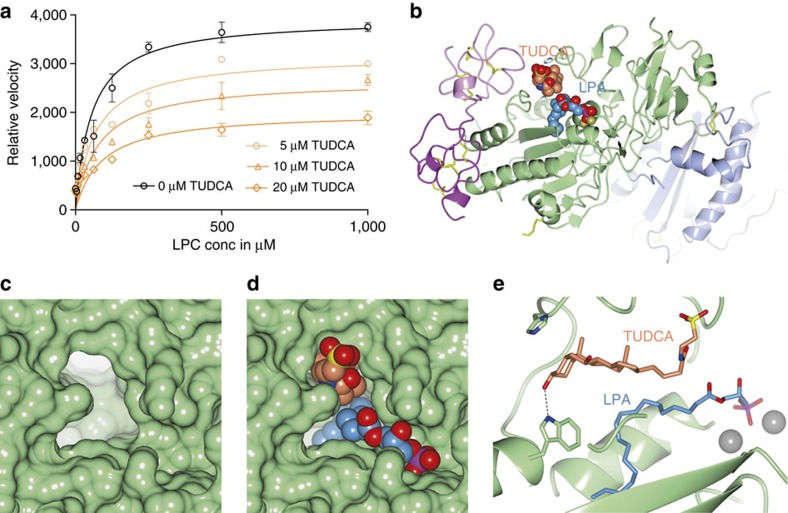
Figure 5. TUDCA acts as a non-competitive inhibitor of LPC hydrolysis.
(a) ATX lysoPLD activity with no inhibitor (black line and symbols) and with three concentrations (conc) of TUDCA (orange lines and symbols) as function of LPC(18:1) substrate conc. Modelling of all data (see the Methods for details) indicate that TUDCA acts as a partial noncompetitive inhibitor, with a Ki of 9±3 μM and residual activity of ∼40% towards LPC. (b) A cartoon of the ATX structure with bound TUDCA (orange carbons) in the tunnel and LPA (blue carbons) in the pocket, both shown as space filling models. (c,d) A zoom-in view showing the molecular surface of ATX at the TUDCA- and LPA-binding sites empty (c) and with bound TUDCA and LPA (d) as space filling models. (e) A zoom-in to a view along the tunnel axis, showing the characteristic L-shaped bile acid ring system and the bound LPA(18:1); the taurine tail has moved away from the active site to make space for the LPA; the active site zincs are visible to the right as grey spheres.