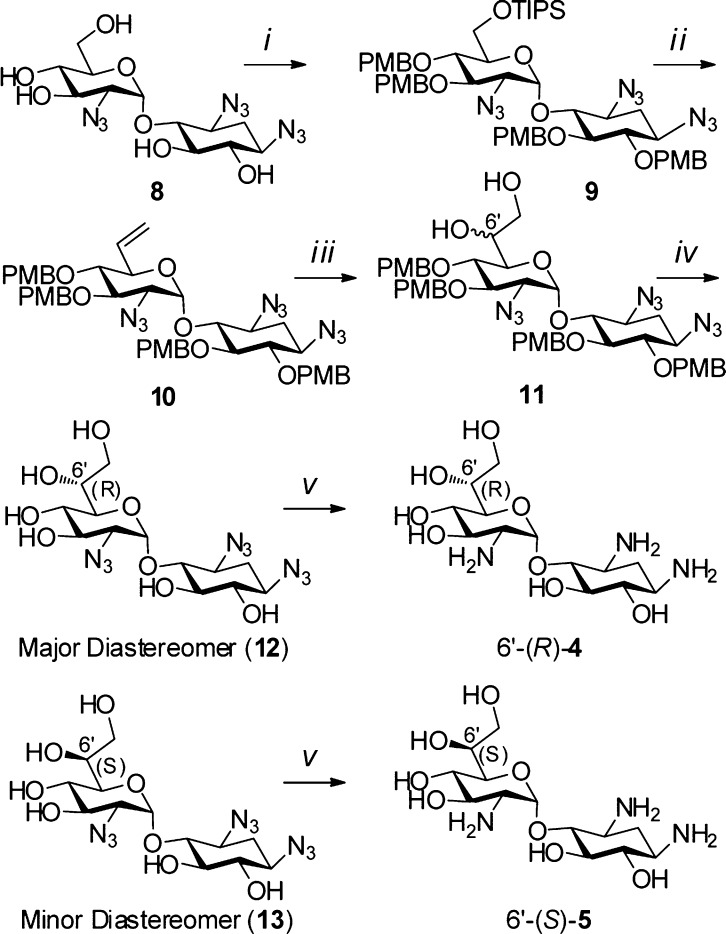
Scheme 1.
Reagents and conditions: (i) (a) TIPSCl, DMF, 4-DMAP, 0–25 °C, 83%; (b) PMBCl, NaH, DMF, 0–25 °C, 84%; (ii) (a) TBAF, THF, 0–25 °C, 88%; (b) IBX, EtOAc, 80 °C, 85%; (c) CH3P(Ph)3I, n-BuLi, THF, 0–25 °C, 56%; (iii) K2OsO4·2H2O, NMO, acetone/H2O/t-BuOH, 93% (3:1 ratio); (iv) (a) DDQ, CH2Cl2/H2O; (b) Ac2O, Py, 0–25 °C, 91% for 2 steps; (c) NaOMe, MeOH, 60%; (v) PMe3, THF, NaOH (0.1 M), 73% [6′-(R)-4]; 78% [6′-(S)-5].