BiTox attenuated A-nociceptor-mediated mechanosensitivity in rat models of chronic pain. Plasma extravasation and keratinocyte proliferation were also inhibited but C-fiber nociception was not impaired.
Keywords: Botulinum toxin, BiTox, Anti-nociception, Inflammatory pain, Neuropathic pain
Abstract
Local injections of botulinum toxins have been reported to be useful not only for the treatment of peripheral neuropathic pain and migraine but also to cause long-lasting muscle paralysis, a potentially serious side effect. Recently, a botulinum A-based molecule (“BiTox”) has been synthesized that retains neuronal silencing capacity without triggering muscle paralysis. In this study, we examined whether BiTox delivered peripherally was able to reduce or prevent the increased nociceptive sensitivity found in animal models of inflammatory, surgical, and neuropathic pain. Plasma extravasation and edema were also measured as well as keratinocyte proliferation. No motor deficits were seen and acute thermal and mechanical nociceptive thresholds were unimpaired by BiTox injections. We found reduced plasma extravasation and inflammatory edema as well as lower levels of keratinocyte proliferation in cutaneous tissue after local BiTox injection. However, we found no evidence that BiTox was transported to the dorsal root ganglia or dorsal horn and no deficits in formalin-elicited behaviors or capsaicin or formalin-induced c-Fos expression within the dorsal horn. In contrast, Bitox treatment strongly reduced A-nociceptor-mediated secondary mechanical hyperalgesia associated with either complete Freund’s adjuvant (CFA)-induced joint inflammation or capsaicin injection and the hypersensitivity associated with spared nerve injury. These results imply that although local release of neuromodulators from C-fibers was inhibited by BiTox injection, C-nociceptive signaling function was not impaired. Taken together with recent clinical data the results suggest that BiTox should be considered for treatment of pain conditions in which A-nociceptors are thought to play a significant role.
1. Introduction
Botulinum neurotoxins (BoNTs), of which there are 7 serotypes, are the most lethal toxins known to man.10,30,31,42 Botulinum neurotoxin/A (Botox), the serotype most commonly used in clinical practice, acts on synaptic transmission by cleavage of the specific target SNAP-25, a member of the SNARE (Soluble N-ethylmaleimide-sensitive factor attachment protein receptor) family of proteins, preventing the correct assembly of the SNARE core complex and thus the fusion of synaptic vesicles with the neuronal presynaptic membrane, inhibiting the release of neurotransmitter.3,44 This occurs both peripherally and within the central nervous system after local injection of the toxin, reversibly silencing synaptic transmission.12,32 Peripheral injections of BoNT/A inhibit exocytosis of acetylcholine from motor nerve terminals causing flaccid muscle paralysis, a property that has made BoNT/A useful for the treatment of many pathological conditions, involving excessive muscle contractions. However, although the specificity of BoNT/A for motor neuron terminals is established, an antinociceptive role of the toxin by binding to nociceptive primary afferents has also been suggested and indeed BoNT/A injections for chronic pain have become a valuable tool in clinical practice. Recent meta-analyses of the clinical literature have recommended local BoNT/A treatment for peripheral neuropathic pain.11 Botulinum neurotoxin/A has also shown effectiveness in the treatment for chronic migraine after injections in the scalp muscles.14,25
There is, however, considerable confusion over the mechanism of BoNT/A-mediated antinociception. For example, a recent study using healthy volunteers reported that intradermal administration of BoNT/A inhibited local protein extravasation and produced a marked decrease in noxious mechanical pain sensitivity although sensitivity to thermal stimuli was unchanged.35 C-nociceptors have been implicated as targets of BoNT/A, as they release neuropeptides substance P (SP) and calcitonin gene-related peptide peripherally after local inflammation causing local vasodilatation and plasma extravasation.16–18,35 Plasma extravasation can be blocked in humans and rodents by local injections of BoNT/A. However, the evidence that BoNT/A also inhibits C-fiber nociceptive function is far less clear and in both clinical and preclinical studies the main effect of BoNT/A seems to be on relieving neuropathic rather than inflammatory pain.2,15,27,39,47,49,50
Recently, a nonparalytic and therefore safer version of the botulinum molecule (BiTox) has been synthesized by “stapling” together the recombinant light chain/translocation domain and receptor-binding domain of BoNT/A. This chimeric molecule exhibited the same “silencing” efficacy on neurons as the native BoNT/A but had significantly reduced paralytic activity a dose-limiting serious side effect of repeated BoNT/A treatment.6,12,13 In this study, we examine the potential antinociceptive effect of BiTox on acute and chronic inflammatory pain as well as in surgical and neuropathic pain models, evaluating the potential of BiTox to replace BoNT/A as a safe treatment for some types of chronic pain.
2. Methods
2.1. Animals
All procedures complied with the UK Animals (Scientific Procedures) Act 1986. Male Sprague Dawley rats (weighing 170-200 g; Central Biological Services, University College London, United Kingdom) were used. Animals were kept in their home cages at 21°C and 55% relative humidity with a light to dark cycle of 12 hours (lights on at 08:00 hours). Food and water were provided ad libitum. All efforts were made to minimize animal suffering and to reduce the number of animals used. A total of 150 animals were used for the study.
2.2. Preparation of BiTox
BoNT/A consists of 3 structurally independent units: the Receptor-binding domain (Rbd), the Translocation domain (Td), and the Light chain (Lc), the last being the “silencing domain” a proteolytic enzyme. Here we use a process called SNARE tagging to bind recombinant proteins together. Thus the light chain translocation domain part of the BoNT/A molecule is linked with SNAP25 and the receptor-binding domain component is linked with synaptobrevin. These recombinant proteins were expressed in bacteria and then mixed with syntaxin protein. Syntaxin, SNAP25, and synaptobrevin bind together to form stable tetrahelical synthetic molecules (see Fig. 2, Ref. 11). Previously, it was shown that the assembled BiTox molecule had unique nonparalyzing properties compared with BoNT/A.11
Briefly, all proteins were expressed in BL21 strain of E. coli as glutathione S-transferase C-terminal fusions cleavable by thrombin. Proteins fused to glutathione S-transferase were purified on Glutathione Sepharose beads (GE Healthcare, Buckinghamshire, United Kingdom) and eluted from beads in 20 mM Hepes, pH 7.3, 100 mM NaCl (Buffer A) using thrombin. Further purification was achieved by gel filtration using a Superdex 200 10/200GLcolumn (GE Healthcare). The BiTox was assembled by mixing the SNARE-tagged proteins with syntaxin peptide (Peptide Synthetics, Hampshire, United Kingdom) for 30 minutes at 20°C, each component at 5 μM concentration. To visualize SNARE assemblies, SDS-PAGE was performed at 4°C, and the gels were stained with Coomassie blue.
Cleaved SNAP25 was derived from SIMA neuroblastoma cells treated with BiTox for 3 days as previously described.6,12 BiTox was generally used at a dose of 200 ng in 50 µL saline unless otherwise stated.
2.3. Drugs delivery and surgery
2.3.1. Intraplantar and intra-ankle joint injections
Under 2% isoflurane anesthesia, a 30 G needle was inserted into the plantar surface of the paw subcutaneously. Fifty microlitres of BiTox (200 ng in 0.9% NaCl solution) or vehicle or 50 μL of CFA (Complete Freund's adjuvant, SIGMA, Gillingham, United Kingdom) were injected for more than 10 to 20 seconds. Fifty microlitres of 2% formalin or 10 μL of 0.3% capsaicin N-vanillylnonanamide (synthetic capsaicin; SIGMA) were injected without anesthesia. For the latter, the animal was lightly restrained in a glove during injection. Ten microlitres CFA was injected into the lateral ankle joint. For dose–response experiments, the concentrations of BiTox used were: 2, 50, 100, and 200 ng. All BiTox injections were given in center of the plantar surface unless otherwise stated.19,26
2.3.2. Spared nerve injury surgery
The spared nerve injury (SNI) was performed as previously described.8 Under 2% isoflurane anesthesia, incision was made on the skin of the thigh and through the biceps femoris muscle exposing the 3 terminal branches of sciatic nerve: the sural, common peroneal, and tibial nerves. The common peroneal and tibial nerves were tightly ligated with 5.0 silk and sectioned distal to the ligation, removing 2 to 4 mm of the distal nerve stump. Muscle and skin were closed in 2 separate layers. Behavioral testing began the day after surgery and continued for 11 days postsurgery.
2.3.3. Plantar Incisional injury
All rats were anesthetized with 2% isoflurane. As described before,4 a 1-cm longitudinal incision was made, with a number 11 scalpel blade, through skin and fascia of the plantar paw, starting 0.5 cm from the proximal edge of heel and extending toward the toes. The plantar muscle was elevated and incised longitudinally. The skin was closed with 5.0 silk sutures.
2.4. Behavioral testing
In all experiments each animal was assigned to one behavioral test only—that is, each animal was never tested in more than one modality, apart from the SNI groups where von Frey testing was followed by the acetone test. The contralateral paw was also tested but no changes in mechanical or thermal thresholds were detected.
2.4.1. Von Frey test
Mechanical sensitivity was assessed using the von Frey filaments (Stoelting, Wood Dale, IL). A series of calibrated von Frey filaments exerting pressure stimuli in the range of 0.04 to 26.0 g were used on the plantar surface of the paw in an ascending order, starting with filament 0.04 g. Before each testing session, animals were placed in individual plastic cages and left to adapt to the environment for at least 20 minutes. The filaments were applied 10 times every 5 seconds and the number of positive responses recorded.19
2.4.2. Pinprick test
The pinprick test was performed as previously described.19 Animals were placed on an elevated wire grid and habituated over a period of 2 to 3 consecutive days by recording a series of baseline measurements. The point of a safety pin was applied to the lateral part of the plantar surface of the paw at intensity sufficient to indent but not to penetrate the skin. The duration of paw withdrawal was recorded with a minimum arbitrary value of 0.5 seconds for a brief response.
2.4.3. Thermal stimulation
Thermal withdrawal thresholds were determined as previously described.19 Animals were allowed to habituate to the apparatus (Ugo Basile, Varese, Italy) for 10 to 15 minutes before testing began. Rats were placed in a clear plastic chamber (18 × 29 × 12.5 cm) with a glass floor and allowed to acclimate to their environment for 5 minutes before testing. During this time, rats initially demonstrated exploratory behavior but subsequently stopped exploring and stood quietly with occasional bouts of grooming. After the acclimation the radiant heat source was positioned under the glass floor directly beneath the hindpaw. A trial commenced when a switch activated the radiant heat source and started an electronic timer. This assay measured the latency in seconds to withdrawal of the hindpaw. The heat intensity was calibrated so that a naive, untreated animal had an average latency of 8 to 10 seconds; to prevent tissue injury, a maximum cut-off value was set at 20 seconds. For each animal, the withdrawal latency was the average of 3 separate determinations, taken with at least 2 minutes between each trial.
2.4.4. Formalin test
The rats were placed into a plexiglas observation chamber (10 × 20 × 24 cm) with a mirror (45° angle) positioned to permit unhindered observation of the rat hindpaws. Formalin was injected subcutaneously into the center of the plantar surface of the left hindpaw. The rat was then replaced in the box, the clock was started and the response was recorded for a period of 1 hour.46 The pain-related behavior was quantified by counting the total number of flinches and shakes, rapid and brief withdrawal movements or flexion of the injected paw, occurring for 1-minute periods from 1 to 5 minutes (Phase I) and, then for 1-min periods at 5-minute intervals during the period from 10 to 60 minutes (Phase II) after formalin injection. Phase I is generally considered the result of chemical activation of nociceptors, whereas Phase II reflects the inflammatory reaction and central processing.46
2.4.5. Acetone test
For assessment of cold sensitivity, the acetone test was used as described previously.8 Approximately, 30 minutes after the von Frey test, mice were tested for paw withdrawal response to a cold stimulus comprising a 50-μL drop of acetone applied to the center of the plantar surface of the hindpaw ipsilateral to the site of injury, avoiding mechanical stimulation of the paw with the syringe. Total time lifting/clutching ipsilateral hindpaw was recorded with an arbitrary maximum cut-off time of 20 seconds. Before testing, animals were habituated over a period of 2 to 3 consecutive days by recording a series of baseline measurements.
2.4.6. Motor function
Motor function was evaluated using the hindpaw digit spreading that occurs when the animal is picked up by the tail or by suspension test in which animals were placed on a wire mesh and the mesh inverted. Time to fall from the mesh or weakness in the hind limb, was noted.
2.5. Plasma extravasation measured with Evans Blue
Under 2% isoflurane anaesthesia, rats were pretreated (3 days) with a single intraplantar (IPLT) injection of BiTox into the hindpaw or vehicle into the plantar surface of contralateral paw. In control groups, the vehicle was delivered into the ipsilateral and contralateral paws. After 3 days, the animals were deeply anesthetized once again with isoflurane and received a tail vein injection of Evans blue (50 mg·kg−1; Sigma-Aldrich). After 15 minutes the animals received 0.3% capsaicin into the ipsilateral (BiTox pre-injected) and 0.3% capsaicin into the saline pre-injected contralateral paw. Rats were sacrificed with CO2 15 minutes later and the ipsi-and contralateral plantar paws were photographed and removed. The dye was extracted with formamide (2 mL per each plantar paw; Sigma-Aldrich) for 72 hours at 37°C. After the incubation, the solvent was submitted for a spectrophotometric analysis of the stain density (620 nm) and calibrated with a standard curve to determine the amount of dye extravasation. Dye concentrations in the ipsilateral and contralateral paws were then compared. The area of blue staining was also measured from photographs of each paw before extraction using an NIH Image J imaging package.
2.6. Bromodeoxyuridine incorporation
Bromodeoxyuridine (BrdU)-labeling was used to measure kerantinocyte proliferation in the basal epidermis. Three different groups of animals were injected with BiTox 32 days, 14 or 3 days before BrdU injection (100 mg·kg−1 in saline, i.p.; SIGMA). The animals were killed 24 hours after BrdU injection by deep barbiturate anaesthesia followed by transcardiac perfusion. The skin tissues were postfixed for 2 hours in 4% paraformaldehyde (PFA), sectioned, and subjected to immunohistochemistry with BrdU antibody (see 2.7 Immunohistochemistry paragraph) after pretreatment with hydrochloric acid 2N for 30 minutes to denature DNA and 0.1 M boric acid for 10 minutes to neutralize the acid. All tissue sections were counterstained with 0.1% DAPI to reveal keratinocytes in the basal layer of the epidermis.22,23
2.7. Immunohistochemistry
Rats were deeply anaesthetized with pentobarbital and perfused transcardially, briefly with saline containing 5000 I.U·mL−1 heparin followed by 4% PFA in 0.1 M phosphate buffer (PB). The glabrous skin of the hindpaw and the spinal cord were dissected out, postfixed in the same PFA solution for 2 hours and transferred into a 30 % sucrose solution in phosphate buffer containing 0.01% azide, for a minimum of 24 hours. Tissues were cut on a freezing microtome at 40 μm. All primary antibodies were used with a tyramide signal amplification and immunofluorescence (as previously detailed26), except for c-Fos when a protocol using diaminobenzidine was used as the final step. Sections were left to incubate with the following primary antibodies: anti-BrdU (1:200; Becton Dickinson, Oxford, United Kingdom; Cat, No.: 347,580), anti-cSNAP25 antibody recognizing the cleaved end of SNAP25 (TRIDEANQ) 1:10,000, and anti-c-Fos (1:10,000; Millipore, Cat, No.: ABE457) for 24 h at room temperature. Appropriate biotinylated secondary antibodies were used at a concentration of 1:400 for 90 minutes. Samples were then incubated with avidin biotin complex (ABC Elite; Vector Lab., CA) (1:250 Vectastain A+1:250 Vectastain B) for 30 minutes followed by a signal amplification step with biotinylated tyramide solution (1:75 for 7 minutes; Perkin Elmer, MA). Finally, sections were incubated with FITC-avidin for 2 hours (1:600). All sections were coverslipped with Gel Mount Aqueous Mounting Medium (Sigma) to protect the fluorescence from fading and stored in dark boxes at 4°C. For c-Fos, immunohistochemistry sections were incubated in anti-c-Fos antibody for 24 hours RT followed by incubation with the biotinylated secondary antibody (1:500) for 2 hours. Samples were then incubated with avidin biotin complex (ABC Elite; Vector Lab.) (1:250 Vectastain A+1:250 Vectastain B) for 1 hours followed by a staining step for 4 minutes in a solution containing 3,3′ diaminobenzidine (DAB Kit; Vector). All sections were left to dry overnight. Finally, the slides were dehydrated and coverslipped with DPX mountant (containing Xylene and DibutylPhtalate). Slides were stored at room temperature.
2.8. Cell counts
To count the number of c-Fos-labeled neurons after formalin or capsaicin stimulation, the superficial dorsal horn was divided into 3 ipsilateral domains: the superficial laminae I–II, laminae III–IV, and the deep laminae V–VII. For each rat, Fos-immunoreactive neurons in all sections were counted (the minimum distance between sections was 200 μm), counts from the 5 most heavily labeled sections were averaged and the mean was used for further statistical analysis. BrdU labelled cells were counted from photomicrographs or directly from the microscope using a 10× objective lens on a Leica DMR microscope and Volocity software (PerkinElmer, Waltham, MA). Ten areas of epidermis were counted per animal over an area of 1 mm2. Sections were at least 200 µm apart. BrdU(+) cells were expressed as BrdU(+) cells per mm2.
2.9. Tissue collection and immunoblotting
Animals were terminally anaesthetized with CO2 3 days after BiTox IPLT injection or its vehicle, and fresh dorsal horn and dorsal root ganglia (DRG) tissues collected. For protein extraction, each sample of dorsal horn or DRG was homogenized in 200 µL lysis buffer (1% Np-40, 20 mM Hepes pH7.4, 100 mM NaCl, 100 mM NaF, 1 mM Na3VO4, 5 mM EDTA with 1× protease inhibitor cocktail (SIGMA); 1× phosphatase inhibitor cocktail (SIGMA)) and incubated on ice for 2 hours. Samples were then centrifuged at 13,000 rpm for 15 minutes and supernatants collected. Total protein concentration was assessed using a bicinchoninic acid protein assay kit (Pierce Biotechnology, IL) before each preparation of protein samples. Samples (10 µg of proteins per well), 5 µL of cleaved SNAP25 (cSNAP25) and uncleaved SNAP25 (uncSNAP25) controls (SIMA neuroblastoma cells) were run on 10% Bis–Tris gels (Biorad Laboratories, CA) for detection of cleaved SNAP25. Proteins were transferred onto a PVDF membrane (Biorad). Membranes were blocked in 10 mM Tris–HCl pH = 7.5, 150 mM NaCl, 0.05% Tween 20 (SIGMA) and 0.24% I-Block (Tropix, MA) and incubated with anti-cSNAP25 primary antibody (1:1000) overnight at 4°C. After washes, a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody was applied for 45 minutes. Horseradish peroxidase activity was visualized by applying PICO developer and using Chemi Doc XRS (Biorad). Membranes were then washed and incubated with glyceraldehyde 3-phosphate dehydrogenase antibody (1:1000, Millipore Merck KGaA, Darmstadt, Germany; Cat, No.: MAB374) for 45 minutes and further processed as described above. Signal intensity was measured using Quantity One software (Biorad).
2.10. Statistical data analysis
Data analysis and statistical comparisons were performed by SPSS (PASW) 18 (IBM) and GraphPad Prism, version 5 for Windows, (GraphPad Software, San Diego, CA). All results are presented as mean±SEM. Each group included 8 to 12 animals in behavioral experiments or 6 animals in immunoblotting approaches. Statistical analysis was performed by 2-way analyses of variance with Bonferroni’s multiple comparison post hoc tests for behavioral data. For immunoblotting, immunohistochemistry results or when 2 groups were compared, Student's t-test was used. For all experiments, a value of P < 0.05 was considered to be statistically significant.
3. Results
3.1. Motor effect of Bitox
No motor deficits were seen after injections of BiTox (200 ng) into the plantar surface of the hindpaw.
3.2. Mechanical and thermal thresholds were not influenced by intraplantar injection of BiTox in naive rats
Mechanical and thermal thresholds were monitored for 5 days after IPLT injection of BiTox and were not different from vehicle treated animals at these time points (Fig. 1).
Figure 1.
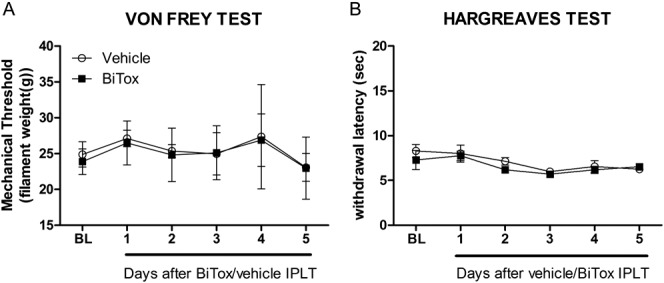
Basal mechanical and thermal thresholds were not influenced by IPLT BiTox injection. Intraplantar (IPLT) injection of 200 ng BiTox had no effect on mechanical thresholds (A) or withdrawal latency to thermal stimulation (B). The measurements were assessed for 5 days before injection to determine baseline pain threshold (BL). Data are presented as means ± SEM, P >0.05. n = 4 animals per group.
3.3. Effects of intraplantar injections of BiTox on inflammatory and plantar incisional pain
3.3.1. BiTox does not reduce the efficacy of intraplantar formalin and capsaicin injection
The response to acute injection with capsaicin or formalin was not affected by BiTox pretreatment. Intraplantar injection with BiTox 3 days before 2% formalin injection did not reduce the number of characteristic flinches in the early and late phases (Fig. 2A). The increased thermal sensitivity seen after capsaicin injections (Fig. 2B) was also unchanged by IPLT BiTox injection given 3 days before 0.3% capsaicin injection. C-Fos expression measured 2 hours after IPLT 0.3% capsaicin or 2% formalin injections was also unchanged by IPLT BiTox injections in all laminae analyzed (Fig. 2C and D).
Figure 2.
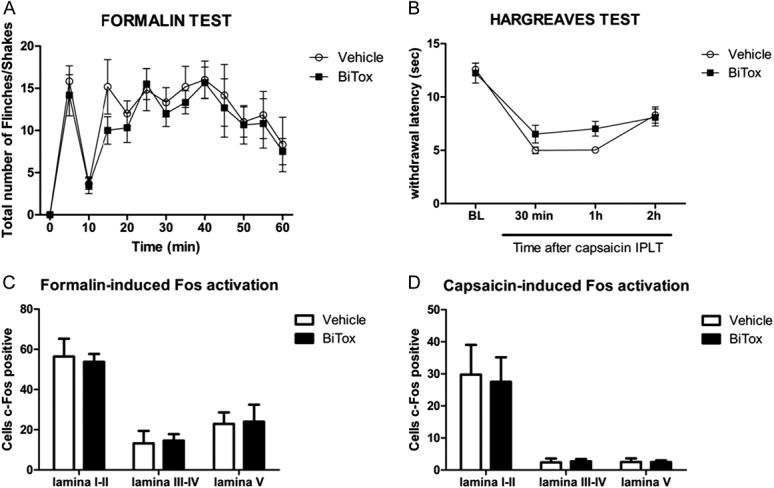
Formalin and capsaicin responses were not reduced by IPLT BiTox injection. (A) First and second phases of flinches measured after 2% formalin injection were unimpaired by IPLT BiTox injection into the ipsilateral hindpaw 3 days previously. (B) Thermal hyperalgesia that develops after 0.3% capsaicin injection is unaffected by BiTox treatment 3 days previously. Total c-Fos counts in dorsal horn laminae I to V after 2% formalin (C) or 0.3% capsaicin injection (D) given 3 days after BiTox injection into the ipsilateral hindpaw. No differences were seen between the number of c-Fos positive cells in the BiTox pretreated group and the vehicle group (A). Data are presented as means ± SEM, P > 0.05. n = 6 animals per group.
3.3.2. Inflammatory mechanical but not thermal hyperalgesia was acutely attenuated by intraplantar injections of BiTox
BiTox treatment injected into the ipsilateral hindpaw 24 hours after IPLT CFA injection transiently reduced the hypersensitivity to mechanical (between day 2 and day 3 post-BiTox, F(1,10) = 38.5, P < 0.001, Fig. 3A) but not thermal stimuli (Fig. 3B). Moreover, CFA-induced paw swelling was reduced (0.675 ± 0.026, t(14) = 2.98, P = 0.010) at 7 days compared with the ipsilateral control group (0.762 ± 0.012) after BiTox treatment.
Figure 3.
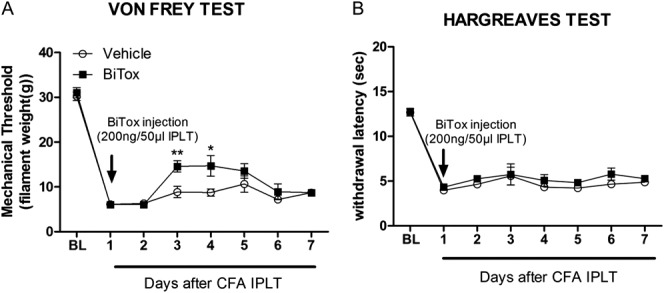
Intraplantar BiTox transiently reduced CFA-induced mechanical but not thermal hypersensitivity. Intraplantar injection of BiTox given 24 hours after IPLT injection of CFA transiently reduced mechanical (A) but not thermal hypersensitivity (B) on days 2 and 3 post-BiTox injection. Baseline pain threshold (BL) was determined for 5 days before injection. Data are presented as means ± SEM, *P > 0.05, **P < 0.01. n = 6 to 8 animals per group.
3.3.3. Incisional sensitivity was acutely inhibited by intraplantar injection of BiTox
Plantar incision increased response to mechanical stimulation around the site of incision measured over 5 days. Twenty-four hours after incision, rats received local BiTox injection or vehicle. Intraplantar BiTox transiently reduced mechanical sensitivity from 1 to 2 days after treatment (between day 2 and day 3, F(1,14) = 6.07, P < 0.027, Fig. 4).
Figure 4.
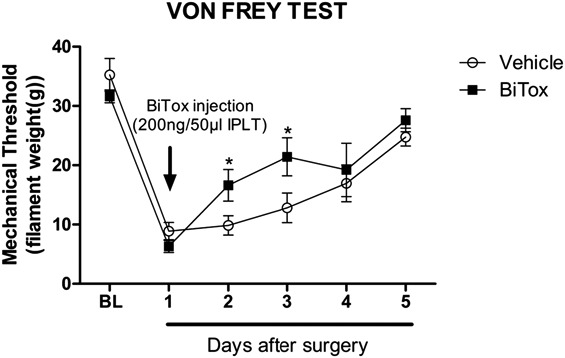
Plantar incision produced mechanical hypersensitivity that was transiently reduced by IPLT BiTox injection. Effect of IPLT injection of BiTox (200 ng) or vehicle on the mechanical withdrawal threshold measured with von Frey filaments in a postsurgical pain model. Measurements were made before injury to determine baseline pain threshold (BL) and 24 hours after the incision, at which time BiTox was injected and behavior followed for 5 days. Mechanical hypersensitivity was reduced on days 2 and 3 after BiTox injection. Data are presented as means ± SEM, *P < 0.05. n = 8 animals per group.
3.4. Intraplantar BiTox injection reduced plasma extravasation
Rats received a single IPLT injection of BiTox into the ipsilateral paw and a single injection of vehicle into the plantar surface of the contralateral paw. After 3 days the animals received a tail vein injection of Evans blue (50 mg·kg−1). Fifteen minutes later 0.3% capsaicin was injected into the ipsilateral (BiTox-treated) and contralateral (vehicle-treated) paws to evoke plasma extravasation. After a further 15-minute period the amount of dye extravasated or the extent of extravasation was measured in paw skin tissue. In the vehicle-injected paw, capsaicin injection induced a massive local extravasation of blue dye. In contrast, intraplantar injection of BiTox induced a reduction in dye extravasation compared with the vehicle-treated paw (t(8) = 3.066, P = 0.015, Fig. 5B; t(10) = 3.011, P = 0.013, Fig. 5C).
Figure 5.
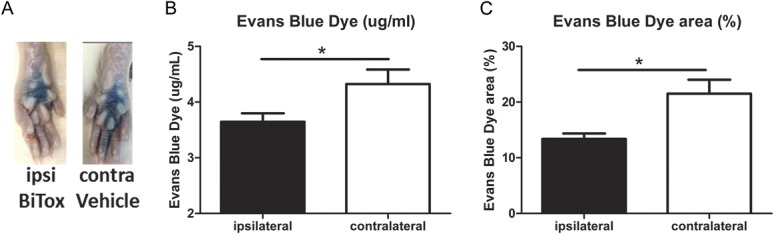
Intraplantar BiTox inhibited capsaicin-induced extravasation. Rats were pretreated with IPLT BiTox (200 ng) or vehicle (contralateral paw) 3 days before plasma extravasation was studied. Tail vein injection of Evans blue (50 mg·kg−1) was given 15 minutes before bilateral IPLT injection of capsaicin. Tissue was collected 15 minutes later. (A) The blue color of hindpaw skin was darker and more extensive in the contralateral vehicle paw compared with BiTox injected paw. Histograms show dye concentration in formamide extract of ipsi- and contralateral paw tissue (B) and the area of Evans Blue extravasation generated from the photographic record (C). Data are expressed as means ± SEM, *P < 0.05. n = 6 animals per group.
3.5. Reduced incorporation of BrdU after intraplantar injection of BiTox
Incorporation of BrdU into proliferating keratinocytes was measured at 4, 15, and 33 days (A, B, and C) after IPLT BiTox injection. In the epidermis of rats pretreated with BiTox 14 and 32 days before systemic BrdU injection, the number of BrdU-labeled cells was reduced compared with the contralateral paw (t(6) = 2.472, P = 0.048 15 days; t(4) = 3.011, P = 0.040 33 days, Fig. 6C). In contrast, there was no significant difference between the paw pretreated (3 days) with IPLT BiTox compared with the contralateral paw (Fig. 6).
Figure 6.
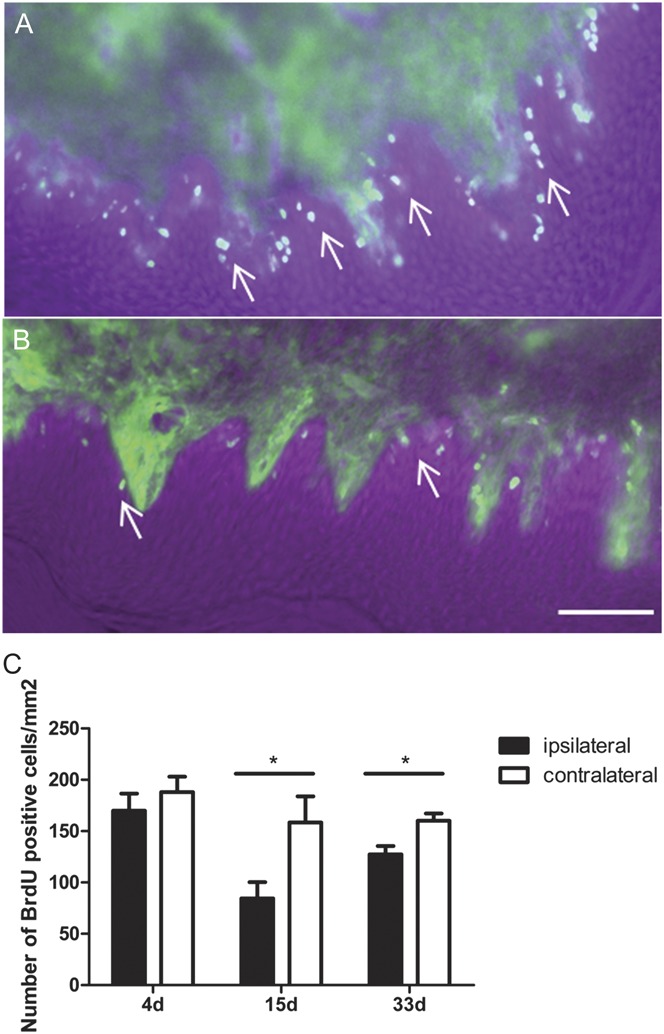
Reduced incorporation of BrdU into keratinocytes after IPLT BiTox treatment. Photomicrographs from sections of cutaneous tissue (A) 14 days after vehicle or (B) BiTox IPLT pretreatment. There was a significant reduction of BrdU positive keratinocytes 14 days or 32 days but not 3 days after BiTox pretreatment (C). Scale bar = 60 μm. Arrows point to BrdU labeled cells in the basal epidermis. Data are presented as means ± SEM, *P < 0.05. n = 3 to 4 animals per group.
3.6. Intraplantar BiTox injection attenuated neuropathic pain and secondary mechanical hyperalgesia
3.6.1. BiTox attenuated neuropathic pain
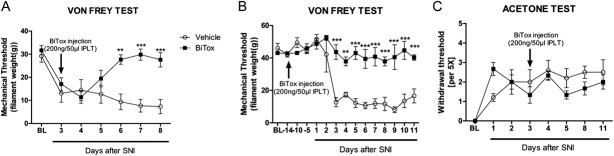
A single IPLT injection of BiTox 72 hours after SNI was sufficient to reduce the mechanical hyperalgesia from 3 days up to 8 days, when the experiment was terminated (F(6.112) = 5.178, P = 0.0001, Fig. 7A). Moreover, BiTox blocks the development of mechanical hyperalgesia when the toxin was injected 2 weeks before SNI (F(14,90) = 7.215, P = 0.0001, Fig. 7B). However, IPLT BiTox did not reduce cold hypersensitivity associated with the model and assessed using the acetone test (Fig. 7C).
Figure 7.
Intraplantar BiTox attenuates SNI-induced mechanical hypersensitivity but not cold hypersensitivity. BiTox (200 ng) or vehicle were injected IPLT 3 days after (A, C) SNI or 14 days before (B) SNI. Mechanical hyperalgesia was attenuated (A, B) but cold allodynia (C) was unaffected by treatment with BiTox injected 3 days after SNI. Baseline pain threshold (BL) was determined for 5 days before surgery or injection. Data are expressed as means ± SEM, **P < 0.01, ***P < 0.001 (A, B), P > 0.05 (C). n = 9 to 10 animals per group.
3.6.2. Secondary mechanical hyperalgesia in CFA model of ankle joint inflammation is attenuated by intraplantar injection of BiTox
BiTox was injected IPLT 3 days after intra-ankle joint injection of CFA. Secondary mechanical hyperalgesia was measured on the plantar surface of the hindpaw. There was a significant reduction in mechanical hyperalgesia between the BiTox group and the vehicle group (F(10,130) = 3.271, P = 0.0008, Fig. 8A) starting at 3 d after CFA treatment but no significant change in CFA-induced edema in the ankle joint (Fig.8B).
Figure 8.
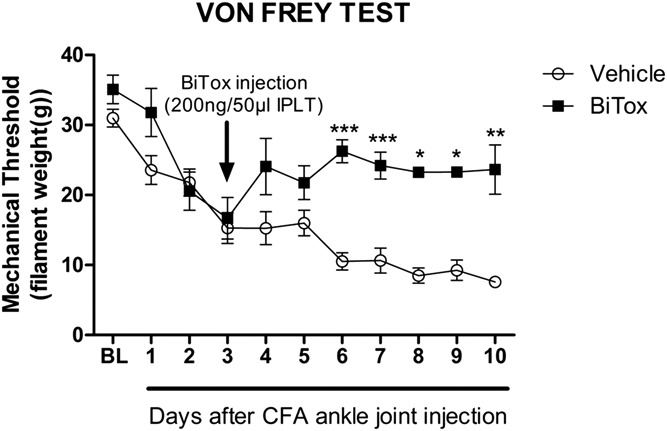
BiTox reduced secondary plantar mechanical hyperalgesia after CFA injection into the ankle. BiTox (200 ng) or vehicle were injected IPLT 3 days after CFA injection into the ankle joint. The mechanical withdrawal threshold was measured with von Frey filaments before treatment as a basal pain threshold (BL) and for 10 days. Intraplantar injection of BiTox attenuated plantar mechanical hypersensitivity after CFA intra-ankle joint injection. Data are presented as means ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001. n = 6 to 8 animals per group.
3.6.3. BiTox attenuated capsaicin-induced secondary mechanical hyperalgesia
Capsaicin injection into the central plantar area of the hindpaw induced thermal hypersensitivity close to the injection site and secondary mechanical hyperalgesia in the zone that surrounds the injection site (Fig. 9A). Secondary mechanical hyperalgesia is thought to be maintained by A-nociceptor signaling amplified centrally by sensitization of dorsal horn neurons (Treede et al., 2000, Magerl et al., 2001). BiTox was delivered in the lateral plantar surface 3 days before capsaicin injection in the central area of the paw. Mechanical stimuli were applied to the area of secondary hyperalgesia area on the lateral plantar surface. BiTox reduced the hypersensitivity only in the area of secondary mechanical hyperalgesia measured with Von Frey filaments (between 30 min and 1 hour, F(1,10) = 27.2, P = 0.001, Fig. 9B) or pin-prick (between 30 minutes and 2 hours, F(3,24) = 8.643, P = 0.0005, Fig. 9C). Thermal hyperalgesia in the area of capsaicin injection and a marker of C-fiber sensitization was not affected by BiTox treatment.
Figure 9.
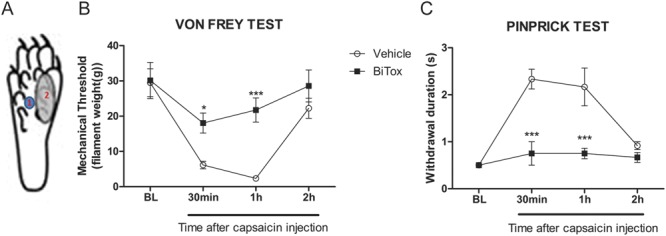
Intraplantar BiTox blocks capsaicin-induced secondary mechanical hyperalgesia. BiTox (200 ng) or vehicle were injected into the lateral plantar surface of the rat hindpaw (A2). Three days later 0.3% capsaicin was injected into the central plantar area (A1) and mechanical secondary hyperalgesia was measured in the lateral zone. Mechanical withdrawal threshold measured with von Frey filaments (B) or withdrawal response duration after pinprick stimulus (C), were attenuated by BiTox pretreatment. The measurements were made before injection (BL) and then 30 minutes, 1 and 2 hours after capsaicin injection. Data are expressed as means ± SEM, *P < 0.05, ***P < 0.001. n = 6 animals per group.
We also used capsaicin-induced secondary hyperalgesia to examine the dose–response of BiTox, using 4 different doses (2, 50, 100, and 200 ng). BiTox was injected in the lateral plantar surface 3 days before capsaicin injection in the central plantar area. Mechanical stimuli were applied to the area of secondary hyperalgesia on the lateral plantar surface. BiTox reduced the hypersensitivity maximally at doses of 100 and 200 ng as measured with Von Frey hairs (F(1,6) = 119.503, P = 0.001, Fig. 10A) or pinprick test (F(1,6) = 51.0, P = 0.001, Fig. 10B).
Figure 10.
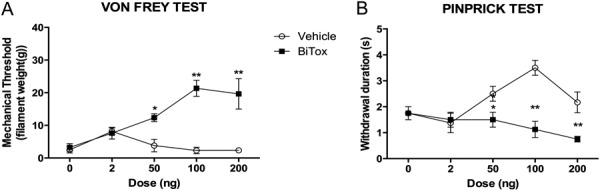
Effect of different doses of IPLT BiTox (2, 50, 100, 200 ng) on capsaicin-induced secondary hyperalgesia. Mechanical withdrawal thresholds measured with von Frey filaments (A) and withdrawal response duration (s) after pinprick stimulation (B). The measurements were assessed before injection and then 30 minutes, 1, and 2 hours after capsaicin injection. Data are expressed as means ± SEM, *P < 0.05, **P < 0.01. n = 4 to 6 animals per group.
3.7. No evidence for transport of active BiTox in peripheral neurons
We investigate whether BiTox was axonally transported to the central nervous system. Dorsal horn and DRG tissue were removed 3 days after IPLT injections of BiTox. Highly sensitive Western blotting failed to demonstrate the presence of cleaved SNAP25 in the dorsal horn and DRG ipsilaterally to the BiTox injection (Fig. 11A and B). SiMa neuroblastoma cells cleaved with BiTox over 3 days were used as a positive control for cSNAP25. The cSNAP25 control, but not uncleaved SNAP25 (uncSNAP25), was clearly recognized by the cSNAP25 antibody. We also used the same antibody directed against cSNAP25 to stain dorsal horn tissue immunohistochemically. BiTox was injected IPLT and tissue for immunohistochemistry collected at 3, 5, 7, and 32 days later. We found no staining for cSNAP25 at any time point. As a positive control we used spinal cord sections from rats injected intrathecally with BiTox and survived 2 hours to 3 days without event. Extensive positive staining was seen in these spinal cords (data not shown).
Figure 11.
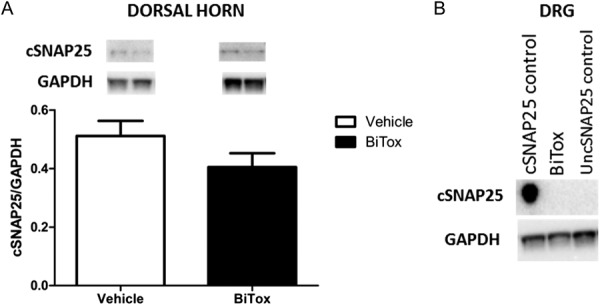
Absence of cleaved SNAP25 in the dorsal horn and DRG after IPLT injection of BiTox. Rats received IPLT injection of BiTox (200 ng) 3 days before sacrifice. The intensity of the bands for cSNAP25 antibody was normalized against the GADPH signal used as internal standard for protein loading in dorsal horn (A). (A and B) show no evidence of cSNAP25 in the DRG or spinal cord 3 days after ipsilateral IPLT BiTox injection. Data are presented as means ± SEM. P > 0.05. n = 6 animals per group.
4. Discussion
BoNT/A has been shown to silence synaptic transmission through specific proteolytic cleavage of the essential neuronal protein, SNAP25.31,42Botulinum neurotoxin/A-mediated synaptic silencing is of long duration and high potency with antinociceptive properties, but in clinical practice there remains the possibility of muscle paralysis. Protein-stapling technology allowed the re-assembly and safe production of BoNT/A (BiTox), from 2 separate fragments.12 The resulting protein was shown to retain synaptic silencing properties between central neurons but failed to block transmission at the neuromuscular junction probably due to steric problems caused by the extensive remodeling of the molecule and its inability to enter the neuromuscular junction. Indeed atomic force microscopy has revealed that the synthetic molecule is now “dumbbell” rather than globular in shape.12 Here we show that a single IPLT injection of BiTox was nonparalysing but effective in attenuating both A-nociceptor-mediated secondary mechanical hyperalgesia and neuropathic pain states modeled in rats. However, BiTox did not reduce C-mediated nociception but did inhibit plasma extravasation and inflammatory edema and reduced keratinocyte proliferation local to the site of toxin injection.
4.1. Primary sensitization of C-fibers is not influenced by intraplantar injection of BiTox
Previous preclinical studies using BoNT/A have yielded conflicting results, particularly concerning effects on C-nociceptors.30 This may have been in part because of various factors, including dose and the use of different pain models but in this study, we found that BiTox had no effect on the thermal hyperalgesia that follows capsaicin treatment or inflammation and thought to reflect the sensitization of C-nociceptors20 or on the induction of c-Fos postsynaptically in superficial dorsal horn neurons.24 Nevertheless previous studies have shown an effect of BoNT/A using injection into the forehead of human subjects, an area innervated by the trigeminal nerve.16,17 Thus we cannot rule out the possibility that primary afferents in different regions of the body respond differentially to BoNT/A or BiTox. However, the mechanical hypersensitivity that develops after surgical incision of the hindpaw or CFA injection is mediated in part by A-fibers4,9,24,34 and it may be that the transient loss of mechanical (but not thermal) hypersensitivity reflects an action on A-nociceptors. It is, however, unclear why the effect should be transient and restricted to mechanical but not thermal sensitivity. It has been shown that the intensity of CFA-provoked inflammatory pain is dependent on proalgesic mediators that are counterbalanced by endogenous analgesic mediators43,45 and it may well be that this balance is transiently shifted towards analgesia when release of neuropeptides from nociceptors is blocked by BiTox.
4.2. BiTox reduces mechanical sensitivity associated with neuropathic pain
A major conclusion from the present study was that BiTox produced a profound long-term reduction in mechanical hyperalgesia in a model of neuropathic pain. A single injection of BiTox was effective in preventing both the development of hyperalgesia and in attenuating the hyperalgesia once it had developed. The analgesic effect of BiTox became significant 3 days after application. Moreover, BiTox showed analgesic effects when it was injected 2 weeks before SNI, preventing the appearance of mechanical hyperalgesia for up to 11 days. A recent meta-analysis of the clinical literature has also recommended local BoNT/A treatment for peripheral neuropathic pain and only at subparalytic doses.14
4.3. A-nociceptors as targets for BiTox
Increased mechanical sensitivity in peripheral neuropathic pain states is thought to require central sensitization of dorsal horn neurons. Central sensitization amplifies A-nociceptor inputs signalling from tissue in and around the site of injury generating secondary mechanical hyperalgesia that is thought to protect the damaged tissue from further damage.28 We tested the hypothesis that A-nociceptors signaling was specifically attenuated by BiTox injections by examining the mechanical hyperalgesia that develops around the site of injury in a number of rat models.26 Plantar mechanical hyperalgesia distance from the site of ankle joint injection of CFA was reduced. We also generated secondary hyperalgesia by injecting capsaicin into the plantar skin. BiTox, delivered in the lateral plantar surface 3 days before capsaicin injection in the central area of the paw, reduced the hypersensitivity only in the surrounding area of mechanical hyperalgesia. This implied that reducing the sensitivity of this subset of A-nociceptors peripherally with a local injection of BiTox, diminished the input to the dorsal horn.
Recent studies using human volunteers have also shown that the area of secondary mechanical hyperalgesia produced by local capsaicin injection was also significantly reduced by BoNT/A, whereas intradermal administration of BoNT-A produced a marked and specific decrease in noxious “pin-prick” mechanical pain sensitivity: sensitivity to low-threshold mechanical and thermal stimuli were largely unchanged.16,17,35 Thus in both human and animal models the results strongly implied that local injection of BoNT/A or BiTox attenuated mechanical hyperalgesia through reduced A-nociceptor signaling.
4.4. Mechanism of action of BiTox
SNAP25 is the only definitely accepted molecular target of BoNT/A action.30 Cleaving of SNAP25 by BoNT/A or BiTox prevents the fusion of neurotransmitter-containing vesicles with the presynaptic membrane and blocks transmitter release.42 Botulinum neurotoxin/A and BiTox are thought to reduce plasma extravasation by inhibiting the release of neuropeptides from subsets of C-nociceptors. The evidence includes reports that have shown that the release of SP from rat DRG cultures was inhibited by BoNT/A38,48 and that SNAP25 was cleaved by addition of BiTox to similar cultures.6 It was also possible that inhibition of cytokine release from mast cells was contributing to reduced extravasation. However, cytokine release from mast cells uses SNAP23,21,37 which is insensitive to BoNT/A.5 In humans, inhibition by BoNT/A was seen within 24 hours after toxin injection but the reduction of A-fiber signaling takes much longer to develop. In rats, the full effect of BiTox on A-nociceptor signaling is significant 3 days postinjection, whereas in humans, loss of pin-prick mechanical hyperalgesia can take up to 3 weeks to develop.35 Two questions arise from these data: is BoNT/A or BiTox binding directly to A-nociceptors and does the delay in the attenuation of mechanical hyperalgesia reflect transport of active botulinum complex to the central terminals of primary afferents within the dorsal horn where the release of neurotransmitter is inhibited by the toxin?
There are some data to suggest that BoNT/A may act through alternative pathways and bind directly to cutaneous A-mechanoreceptors although the mechanisms are unclear.30,35 Nevertheless, if the only site of action of BiTox is on release of neuropeptides from C-nociceptors, this raises the possibility that C-fibers may be having a direct or more likely an indirect effect on maintaining A-nociceptor function peripherally within cutaneous tissues perhaps through trophic effects necessary to maintain healthy tissue. Release of neuropeptides and other molecules from C-fibers has been reported to have important effects on the proliferation of keratinocytes in the epidermis.41 Neuropeptides, particularly calcitonin gene-related peptide but not SP, released from the C-fiber terminals were found to induce keratinocytes proliferation and increase skin thickness and the close contact of peptidergic nerve fibers with other cell types, including mast cells and Langerhan cells, suggests a functional interaction. Previous studies have also shown that peripheral nerve section in rats results in a rapid reduction of keratinocyte proliferation maximal at 3 days.22,23 Here we show that blocking release from C-fibers reduced keratinocyte proliferation at 15 and 33 days after Bitox treatment supporting the hypothesis that C-fibers actively maintain the integrity of undamaged cutaneous tissues. Large numbers of cytokines and chemokines have been identified in skin tissue7 but it remains to be seen if any of these is involved in maintaining A-fiber function. There is no doubt that this population of A-nociceptors has a unique molecular profile, expressing high levels of the activated kinase mTOR (the mammalian target of rapamycin) and being capsaicin insensitive.33,36 Indeed, local injections of mTOR inhibitor rapamycin into the hindpaw will also attenuate the mechanical hypersensitivity associated with peripheral neuropathy and secondary hyperalgesia. It may well be that factors released by cutaneous tissues act to maintain A-fibers through the mTOR complex 1 pathway.33
A second explanation for the delay in the attenuation of mechanical sensitivity after BiTox or BoNT/A injection is that this represents the time taken for active toxin to be transported in primary afferent fibers to the dorsal horn. Indeed there is some evidence that trigeminal nerve fibers may transport BoNT/A.29,39 This would potentially result in the inhibition of neurotransmitter release and block the induction of central sensitization. However, although there is good evidence to suggest that active BoNT/A is transported in central axons,1,40 evidence for transport of active BoNT/A in peripheral nerves is controversial. In the present study, we found no evidence that BiTox was transported centrally using either Western blotting or immunohistochemistry.
5. Conclusions
In summary, we present evidence to show that local BiTox injection has a strong antinociceptive effect predominantly on A-nociceptors and suggest that the use of BiTox, a novel botulinum conjugate, should be targeted specifically to pain conditions in which A-nociceptors are thought to play a significant role.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supported by MRC grant MR/K022539/1 and by CARIPLO Project “Red Drug Train” (2012-2015).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci 2008;28:3689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bach-Rojecky L, Dominis M, Lackovic Z. Lack of anti-inflammatory effect of botulinum toxin type A in experimental models of inflammation. Fundam Clin Pharmacol 2008;22:503–9. [DOI] [PubMed] [Google Scholar]
- [3].Binz T, Sikorra S, Mahrhold S. Clostridial neurotoxins: mechanism of SNARE cleavage and outlook on potential substrate specificity reengineering. Toxins (Basel) 2010;2:665–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brennan TJ. Pathophysiology of postoperative pain. PAIN 2011;152(3 suppl):S33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen F, Foran P, Shone CC, Foster KA, Melling J, Dolly JO. Botulinum neurotoxin B inhibits insulin-stimulated glucose uptake into 3T3-L1 adipocytes and cleaves cellubrevin unlike type A toxin which failed to proteolyze the SNAP-23 present. Biochemistry 1997;36:5719–28. [DOI] [PubMed] [Google Scholar]
- [6].Darios F, Niranjan D, Ferrari E, Zhang F, Soloviev M, Rummel A, Bigalke H, Suckling J, Ushkaryov Y, Naumenko N, Shakirzyanova A, Giniatullin R, Maywood E, Hastings M, Binz T, Davletov B. SNARE tagging allows stepwise assembly of a multimodular medicinal toxin. Proc Natl Acad Sci U S A 2010;107:18197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dawes JM, Antunes-Martins A, Perkins JR, Paterson KJ, Sisignano M, Schmid R, Rust W, Hildebrandt T, Geisslinger G, Orengo C, Bennett DL, McMahon SB. Genome-wide transcriptional profiling of skin and dorsal root ganglia after ultraviolet-B-induced inflammation. PLoS One 2014;9:e93338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. PAIN 2000;87:149–58. [DOI] [PubMed] [Google Scholar]
- [9].Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 2006;26:1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dolly JO, Aoki KR. The structure and mode of action of different botulinum toxins. Eur J Neurol 2006;13(suppl 4):1–9. [DOI] [PubMed] [Google Scholar]
- [11].Ferrari E, Gu C, Niranjan D, Restani L, Rasetti-Escargueil C, Obara I, Geranton SM, Arsenault J, Goetze TA, Harper CB, Nguyen TH, Maywood E, O'Brien J, Schiavo G, Wheeler DW, Meunier FA, Hastings M, Edwardson JM, Sesardic D, Caleo M, Hunt SP, Davletov B. Synthetic self-assembling clostridial chimera for modulation of sensory functions. Bioconjug Chem 2013;24:1750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ferrari E, Maywood ES, Restani L, Caleo M, Pirazzini M, Rossetto O, Hastings MH, Niranjan D, Schiavo G, Davletov B. Re-assembled botulinum neurotoxin inhibits CNS functions without systemic toxicity. Toxins (Basel) 2011;3:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ferrari E, Soloviev M, Niranjan D, Arsenault J, Gu C, Vallis Y, O'Brien J, Davletov B. Assembly of protein building blocks using a short synthetic peptide. Bioconjug Chem 2012;23:479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Foster L, Clapp L, Erickson M, Jabbari B. Botulinum toxin A and chronic low back pain: a randomized, double-blind study. Neurology 2001;56:1290–3. [DOI] [PubMed] [Google Scholar]
- [16].Gazerani P, Pedersen NS, Drewes AM, Arendt-Nielsen L. Botulinum toxin type A reduces histamine-induced itch and vasomotor responses in human skin. Br J Dermatol 2009;161:737–45. [DOI] [PubMed] [Google Scholar]
- [17].Gazerani P, Pedersen NS, Staahl C, Drewes AM, Arendt-Nielsen L. Subcutaneous Botulinum toxin type A reduces capsaicin-induced trigeminal pain and vasomotor reactions in human skin. PAIN 2009;141:60–9. [DOI] [PubMed] [Google Scholar]
- [18].Geppetti P, Nassini R, Materazzi S, Benemei S. The concept of neurogenic inflammation. BJU Int 2008;101(suppl 3):2–6. [DOI] [PubMed] [Google Scholar]
- [19].Geranton SM, Jimenez-Diaz L, Torsney C, Tochiki KK, Stuart SA, Leith JL, Lumb BM, Hunt SP. A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci 2009;29:15017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med 2010;16:1248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hepp R, Puri N, Hohenstein AC, Crawford GL, Whiteheart SW, Roche PA. Phosphorylation of SNAP-23 regulates exocytosis from mast cells. J Biol Chem 2005;280:6610–20. [DOI] [PubMed] [Google Scholar]
- [22].Hsieh ST, Lin WM. Modulation of keratinocyte proliferation by skin innervation. J Invest Dermatol 1999;113:579–86. [DOI] [PubMed] [Google Scholar]
- [23].Huang IT, Lin WM, Shun CT, Hsieh ST. Influence of cutaneous nerves on keratinocyte proliferation and epidermal thickness in mice. Neuroscience 1999;94:965–73. [DOI] [PubMed] [Google Scholar]
- [24].Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 1987;328:632–4. [DOI] [PubMed] [Google Scholar]
- [25].Jackson JL, Kuriyama A, Hayashino Y. Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults: a meta-analysis. JAMA 2012;307:1736–45. [DOI] [PubMed] [Google Scholar]
- [26].Jimenez-Diaz L, Geranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP. Local translation in primary afferent fibers regulates nociception. PLoS One 2008;3:e1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kramer HH, Angerer C, Erbguth F, Schmelz M, Birklein F. Botulinum Toxin A reduces neurogenic flare but has almost no effect on pain and hyperalgesia in human skin. J Neurol 2003;250:188–93. [DOI] [PubMed] [Google Scholar]
- [28].Magerl W, Fuchs PN, Meyer RA, Treede RD. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain 2001;124:1754–64. [DOI] [PubMed] [Google Scholar]
- [29].Matak I, Bach-Rojecky L, Filipovic B, Lackovic Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience 2011;186:201–7. [DOI] [PubMed] [Google Scholar]
- [30].Matak I, Lackovic Z. Botulinum toxin A, brain and pain. Prog Neurobiol 2014;119–120:39–59. [DOI] [PubMed] [Google Scholar]
- [31].Meunier FA, Schiavo G, Molgo J. Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. J Physiol Paris 2002;96:105–13. [DOI] [PubMed] [Google Scholar]
- [32].Montal M. Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem 2010;79:591–617. [DOI] [PubMed] [Google Scholar]
- [33].Obara I, Geranton SM, Hunt SP. Axonal protein synthesis: a potential target for pain relief? Curr Opin Pharmacol 2012;12:42–8. [DOI] [PubMed] [Google Scholar]
- [34].Obara I, Tochiki KK, Geranton SM, Carr FB, Lumb BM, Liu Q, Hunt SP. Systemic inhibition of the mammalian target of rapamycin (mTOR) pathway reduces neuropathic pain in mice. PAIN 2011;152:2582–95. [DOI] [PubMed] [Google Scholar]
- [35].Paterson K, Lolignier S, Wood JN, McMahon SB, Bennett DL. Botulinum toxin-A treatment reduces human mechanical pain sensitivity and mechanotransduction. Ann Neurol 2014;75:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Price TJ, Geranton SM. Translating nociceptor sensitivity: the role of axonal protein synthesis in nociceptor physiology. Eur J Neurosci 2009;29:2253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Puri N, Roche PA. Ternary SNARE complexes are enriched in lipid rafts during mast cell exocytosis. Traffic 2006;7:1482–94. [DOI] [PubMed] [Google Scholar]
- [38].Purkiss J, Welch M, Doward S, Foster K. Capsaicin-stimulated release of substance P from cultured dorsal root ganglion neurons: involvement of two distinct mechanisms. Biochem Pharmacol 2000;59:1403–6. [DOI] [PubMed] [Google Scholar]
- [39].Ramachandran R, Yaksh TL. Therapeutic use of botulinum toxin in migraine: mechanisms of action. Br J Pharmacol 2014;171:4177–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Restani L, Antonucci F, Gianfranceschi L, Rossi C, Rossetto O, Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A). J Neurosci 2011;31:15650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Roggenkamp D, Kopnick S, Stab F, Wenck H, Schmelz M, Neufang G. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J Invest Dermatol 2013;133:1620–8. [DOI] [PubMed] [Google Scholar]
- [42].Salinas S, Schiavo G, Kremer EJ. A hitchhiker's guide to the nervous system: the complex journey of viruses and toxins. Nat Rev Microbiol 2010;8:645–55. [DOI] [PubMed] [Google Scholar]
- [43].Sauer RS, Hackel D, Morschel L, Sahlbach H, Wang Y, Mousa SA, Roewer N, Brack A, Rittner HL. Toll like receptor (TLR)-4 as a regulator of peripheral endogenous opioid-mediated analgesia in inflammation. Mol Pain 2014;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev 2000;80:717–66. [DOI] [PubMed] [Google Scholar]
- [45].Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med 2003;9:1003–8. [DOI] [PubMed] [Google Scholar]
- [46].Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. PAIN 1992;51:5–17. [DOI] [PubMed] [Google Scholar]
- [47].Tugnoli V, Capone JG, Eleopra R, Quatrale R, Sensi M, Gastaldo E, Tola MR, Geppetti P. Botulinum toxin type A reduces capsaicin-evoked pain and neurogenic vasodilatation in human skin. PAIN 2007;130:76–83. [DOI] [PubMed] [Google Scholar]
- [48].Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 2000;38:245–58. [DOI] [PubMed] [Google Scholar]
- [49].Xiao L, Cheng J, Zhuang Y, Qu W, Muir J, Liang H, Zhang D. Botulinum toxin type A reduces hyperalgesia and TRPV1 expression in rats with neuropathic pain. Pain Med 2013;14:276–86. [DOI] [PubMed] [Google Scholar]
- [50].Yuan RY, Sheu JJ, Yu JM, Chen WT, Tseng IJ, Chang HH, Hu CJ. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology 2009;72:1473–8. [DOI] [PubMed] [Google Scholar]