Abstract
Background
Relevant to trauma induced coagulopathy (TIC) diagnostics, microfluidic assays allow controlled hemodynamics for testing of platelet and coagulation function using whole blood.
Methods
Hemodilution or hyperfibrinolysis was studied under flow with modified healthy whole blood. Furthermore, platelet function was also measured using whole blood from trauma patients admitted to a Level 1 Trauma center. Platelet deposition was measured with PPACK-inhibited blood perfused over collagen surfaces at a wall shear rate of 200 s−1, while platelet/fibrin deposition was measured with corn trypsin inhibitor (CTI)-treated blood perfused over TF/collagen.
Results
In hemodilution studies, PPACK-treated blood displayed almost no platelet deposition when diluted to 10% Hct with saline, platelet poor plasma (PPP), or platelet rich plasma (PRP). Using similar dilutions, platelet/fibrin deposition was essentially absent for CTI-treated blood perfused over TF/collagen. To mimic hyperfibrinolysis during trauma, exogenous tPA (50 nM) was added to blood prior to perfusion over TF/collagen. At both venous and arterial flows, the generation and subsequent lysis of fibrin was detectable within 6 min, with lysis blocked by addition of the plasmin inhibitor, ε-aminocaproic acid. Microfluidic assay of PPACK-inhibited whole blood from trauma patients revealed striking defects in collagen response and secondary platelet aggregation in 14 of 21 patients, while platelet hyperfunction was detected in 3 of 20 patients.
Conclusions
Rapid microfluidic detection of (i) hemodilution-dependent impairment of clotting, (ii) clot instability due to lysis, (iii) blockade of fibrinolysis, or (iv) platelet dysfunction during trauma may provide novel diagnostic opportunities to predict TIC risk.
Level of Evidence
Level IV
Study type
Diagnostic Test
Keywords: Platelet function, bleeding, flow assays, diagnostics, microfluidics
BACKGROUND
Trauma is the leading cause of death in people under the age of 36 years old (1). Many severely injured patients exhibit trauma induced coagulopathy (TIC), a hemorrhagic state that accounts for 40% of trauma deaths (1). TIC is multifactorial and associated with tissue injury, inflammation, shock, hemodilution, acidosis, hypoxia, and hypothermia (1). Tissue injury and shock result in hyperfibrinolysis due to the acute release of tissue plasminogen activator (tPA) from endothelial cells. Systemic fibrinolysis results in fibrinogen consumption and limits clot formation and stability at the site of vascular injury, resulting in increased bleeding risk (2). Furthermore, blood loss followed by resuscitation with colloids or packed red blood cells (PRBCs) leads to the hemodilution of clotting factors.
Most research in TIC has focused on coagulation factors and proteases, with the role of platelet function during trauma not as well studied (3,4). Platelet function studies of trauma patients have been difficult to implement due to the technical complexities of current platelet function tests. Although recent advances in platelet aggregometry and thromboelastography (TEG) have enabled assessment of platelet function and clot strength (5–9). These techniques, however, are closed systems lacking flow or presenting poorly defined flow fields.
Microfluidic systems are open systems where blood flows over a zone of defined procoagulant surface, thereby recreating the unique spatial and compositional attributes of blood clotting found in vivo (10). Microfluidic technology in conjunction with micropatterning techniques enable low volume and high throughput testing of platelet function and fibrin generation over a range of physiological shear stresses (11–15). Microfluidic whole blood assays have been previously used to evaluate platelet and clotting function in hemophiliacs and healthy donors taking antiplatelet therapeutics (10,16–18).
In regards to current resuscitation strategies, the administration of PRBCs with or without fresh frozen plasma (FFP) and the optimal FFP:PRBCs ratio remain active areas of investigation (19). Platelets may serve as the third component of resuscitation strategy (19,20). PRBC administration not only increases hemoglobin, but also contributes biorheologically by driving platelet margination towards the vessel wall.
In this study, we applied microfluidic technology to investigate resuscitation-driven hemodilution, hyperfibrinolysis, and plasmin-inhibitor therapy, all topics relevant to TIC risk and treatment. Additionally, we evaluated platelet function under flow using whole blood from trauma patients. In our ex vivo microfluidic studies, we measured severe decreases in platelet adhesion and fibrin accumulation on TF/collagen surfaces with resuscitation-driven hemodilution. Our microfluidic model was also able to rapidly detect hyperfibrinolysis and its inhibition under flow. Finally, we found significant loss of platelet function under flow in 14 of 20 trauma patients. These results illustrate the first implementation of microfluidic technology as a diagnostic platform to assess TIC.
METHODS
Microfluidic evaluation of hemodilution and hyperfibrinolysis
Following approval from the Internal Review Board approval at the University of Pennsylvania, healthy donors (n = 15) were recruited to donate whole blood using standard phlebotomy techniques. Donors were required to refrain from all oral medications for 7 days and abstain from alcohol for 48 hours prior to donation. Blood was drawn into corn trypsin inhibitor (CTI, Haematologic Technologies, Essex Junction, VT, 40 µg/ml final concentration) or FPR-chloromethylketone (PPACK, Haematologic Technologies, Essex Junction, VT, 100 µM final concentration). CTI inhibits the contact pathway for studies of surface-triggered coagulation while PPACK inhibits thrombin generation in order to examine platelet function in the absence of thrombin in vitro. Blood samples were treated with fluorescently conjugated anti-CD61a antibody (clone VI-PL2, Becton Dickson, Franklin Lakes, NJ, 0.125 µg/ml final concentration) to label platelets and fluorescently conjugated fibrinogen (Invitrogen, Life Technologies, Carlsbad, CA, 75 µg/ml) to label fibrin(ogen) 5 min prior to initiation of flow assays. All healthy donor microfluidic experiments were completed within 45 min of phlebotomy.
Microfluidic fabrication methods and device specifications were previously described (11,17,21). Microfluidic channels ran perpendicularly over a 250 µm wide strip of patterned equine fibrillar collagen type I (Chronopar, Chronolog) or Tissue factor (TF) bearing collagen type I surfaces (Dade Innovin, Siemens Healthcare USA, Malvern, PA). Epifluorescent microscopy and image acquisition were performed in real-time as previously described (10,17). Dilution of the hematocrit to simulate resuscitation-induced hemodilution was achieved with exogenous addition of HEPES buffered saline (HBS, 20 mM HEPES, 160 mM NaCl, pH 7.5), donor specific platelet poor plasma (PPP) or platelet rich plasma (PRP). Isolation of PRP or PPP was previously described (17,22). HBS, PPP or PRP was added in 1:3, 1:1, 3:1 ratio to whole blood to obtain Hct levels of 30%, 20%, 10% respectively 5 min prior to initiation of the flow assay. Fibrinolysis was promoted by exogenously adding 10X stock solutions of tPA (abcam, Cambridge, MA, 0-50 nM). Blood samples were perfused at an initial venous wall shear rate of 200 s−1 or arterial wall shear rate of 1222 s−1 in a previously designed pressure relief mode for 20 min (12). Platelet and fibrin accumulation analysis was completed as previously described (17). Statistical significance was assessed using a two-tailed Student's t-test.
Microfluidic Assessment of Trauma Patient Platelet Function
Following Institutional Review Board approval, blood was collected from trauma patients (n = 20) who had sustained injuries requiring evaluation at the Hospital of University of Pennsylvania (HUP) Level 1 Trauma Center. Exclusion criteria included failure to obtain an initial blood draw and death within 24 hr of admission. Clinical data and relevant patient characteristics were recorded (Table 1). All trauma patients had Hct levels and platelet counts within physiologic ranges (Table 1). A blood sample was drawn upon patient arrival using 21-guage or larger needle into a 10 ml plastic syringe (Becton Dickson, Franklin Lakes, NJ) containing no anticoagulant. Blood samples were aliquoted into vacationers for clinical assays and residual blood was transferred into a single vacutainer containing 100 µM PPACK. The effects of this collection method and the delay between phlebotomy and inhibition by PPACK (average ~ 3 min) were assessed and found to have negligible effects on platelet activation and thrombin generation (Supplemental Fig. 6 & Fig. 7).
TABLE 1.
Twenty trauma patients were examined in microfluidic assays with PPACK-inhibited whole blood perfusion over collagen. Trauma patient characteristics and clinical data are as reported, blood products post microfluidic testing were administered within the first hour of evaluation by Hospital of University of Pennsylvania (HUP) Level 1 Trauma Center. Medications altering coagulation, blood product use, pre-existing conditions, Hct, platelet count, Injury Severity Score (ISS), diagnosis of traumatic brain injury (TBI), PT, PTT, INR, blood alcohol level, and blood products post microfluidic testing are as reported. (MCC, motorcycle crash; MVC, motor vehicle crash; GSW: gun shot wound; SW, stab wound.)
| Patient # |
Mechanism | Age | Medication affecting plt function |
Blood products prior to admission |
Pre-exisiting conditions | Hct | Platelets (103/μL) |
ISS | TBI | PTT (secs) |
PT (secs) |
INR | Blood alcohol (mg/dL) |
Blood products post microfluidic testing |
24 h Mortality |
In - hospital mortality |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | MCC | 23 | No | No | None | 36 | 238 | 22 | No | 27.3 | 15.1 | 1.3 | <10 | PRBCS | N | N |
| 2 | 4 | Stroke | 83 | No | No | hypertension | 40 | 251 | 0 | No | 22.3 | 11.7 | 0.9 | 72 | FPP & Platelets |
N | N |
| 3 | 5 | Fall | 84 | Aspirin, Plavix |
No | peripheral vascular disease, diabetes, hypertension, chronic kidney disease |
36 | 142 | 11 | Yes | 29.8 | 13.2 | 1.1 | 0 | None | N | N |
| 4 | 6 | MVC | 60 | coumadin | No | DVT, depression, | 39 | 186 | 27 | Yes | 27.2 | 19.2 | 1.7 | 263 | None | N | N |
| 5 | 7 | MCC | 25 | No | 4U PRBC | None | 42 | 126 | 43 | Yes | 25.5 | 14.5 | 1.2 | <10 | PRBCS | N | N |
| 6 | 9 | Fall | 68 | No | No | hypertension, diabetes | 34 | 171 | 30 | Yes | 44 | 15.3 | 1.3 | <10 | PRBCS | N | N |
| 7 | 11 | Fall | 29 | No | No | Haemophilia A | 36 | 281 | 0 | No | 81.6 | 13.7 | 1.1 | <10 | None | N | N |
| 8 | 13 | Assault | 47 | No | No | schizophrenia, etoh abuse, | 40 | 207 | 34 | Yes | 26 | 14.5 | 1.2 | 178 | PRBCS & FFP |
N | N |
| 9 | 15 | Fall | 88 | No | No | coronary artery disease, hypertension |
40 | 163 | 5 | No | 27.8 | 14.9 | 1.2 | 0 | None | N | N |
| 10 | 17 | GSW | 31 | No | No | None | 47 | 320 | 26 | No | X | X | X | 139 | None | N | N |
| 11 | 19 | Fall | 47 | Lovenox, Aspirin |
No | Hypertension, cardiomyopathy, |
40 | 295 | 1 | No | 26.9 | 13.2 | 1.1 | 0 | None | N | N |
| 12 | 20 | Fall | 77 | No | No | Atrial Fib | 36 | 142 | 2 | No | 20.3 | 12.4 | 1 | <10 | None | N | N |
| 13 | 22 | MVC | 28 | Heroin | No | asthma | 43 | 188 | 2 | No | 28.4 | 13.5 | 1.1 | <10 | None | N | N |
| 14 | 24 | SW | 27 | No | No | asthma | 39 | 224 | 10 | No | 25.5 | 13.7 | 1.1 | <10 | None | N | N |
| 15 | 26 | MVC | 31 | No | No | alcohol abuse | 49 | 245 | 2 | No | 21.9 | 13.9 | 1.2 | 439 | None | N | N |
| 16 | 28 | Fall | 80 | Aspirin | No | hypertension, hyperlipidemia |
38 | 58 | 2 | No | 29.5 | 14.1 | 1.2 | 0 | None | N | N |
| 17 | 31 | Fall | 55 | No | No | hypertension | 33 | 247 | 27 | No | 28.9 | 14.3 | 1.2 | <10 | PRBC, FFP, & Platelets |
N | N |
| 18 | 34 | MVC | 26 | No | 1U PRBC | asthma | 29 | 93 | 26 | No | 31.2 | 15.2 | 1.3 | 0 | PRBCs, FFP, & Platelets |
N | N |
| 19 | 35 | SW | 45 | No | No | Seizure disorder, on dilantin | 38 | 193 | 1 | No | 30.9 | 12.8 | 1 | 307 | None | N | N |
| 20 | 36 | Pedestrian Struck |
50 | No | No | None | 37 | 348 | 1 | No | 30.1 | 13.6 | 1.1 | 96 | None | N | N |
PPACK treated blood samples were treated with fluorescently conjugated anti-CD61a antibody (clone VI-PL2, Becton Dickson, Franklin Lakes, NJ, 0.125 µg/ml final concentration) to label platelets and anti-human CD62P (P-Selectin) antibody (BioLegend, San Diego, CA, 4 µg/ml final concentration) to stain for P-Selectin. Blood samples were subsequently treated with either HBS or 100X stock solutions of the indicated antagonists. MRS 2179 (2'-deoxy-N6-methyladenosine 3', 5'-bisphosphate ammonium salt (MRS 2179, Tocris Bioscience, Minneapolis, MN), S-Nitrosoglutathione (GSNO, Santa Cruz Biotechnology, Dallas TX), and iloprost (Tocris Bioscience) were the antagonists used and dissolved at 0.1 mM, 10 mM, 0.2 mM in HBS respectively. Whole blood perfusion in microfluidic devices started within 10 min of blood collection in constant flow mode at 100 s−1.
RESULTS
Hemotocrit reduction: dilution reduces platelet deposition on collagen (no thrombin) and platelet-fibrin accumulation on TF/collagen
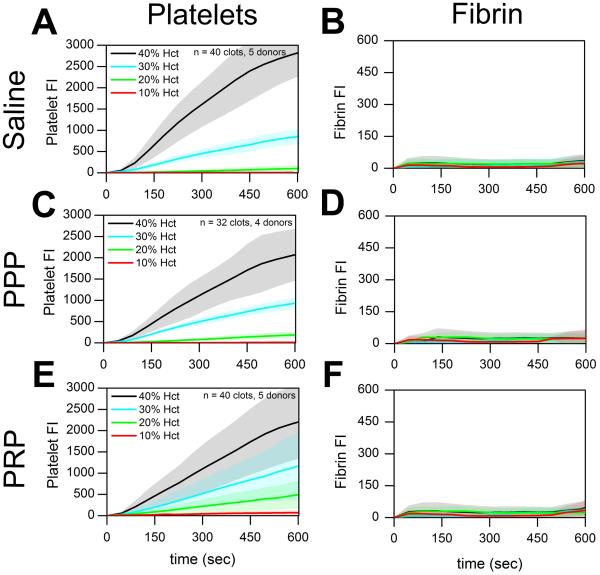
The effect of hematocrit dilution on platelet deposition was evaluated by ex vivo dilution of healthy whole blood with either saline, PPP, or PRP (Supplemental Fig. 1). In the absence of thrombin with PPACK-treated whole blood at 200 s−1, platelet deposition was strongly inhibited (p < 0.01) by hemodilution with saline, PPP, or PRP (Fig. 1A,C,E). As expected in the flow assay over collagen, fibrin generation was dependent on thrombin production and was negligible in PPACK-inhibited whole blood in the absence of surface-patterned TF on collagen (Fig. 1B, D, F).
Figure 1. Platelet accumulation on collagen in healthy donors following Hct dilution in the absence of thrombin.
(A & B), Platelet and fibrin fluorescence intensities vs. time with saline dilution of Hct. (C & D), Platelet and fibrin fluorescence vs. time with PPP dilution of Hct. (E & F), Platelet and fibrin fluorescence intensities vs. time with PRP dilution of Hct. Shaded traces are the mean and standard deviation of 10 clotting events from 5 donors.
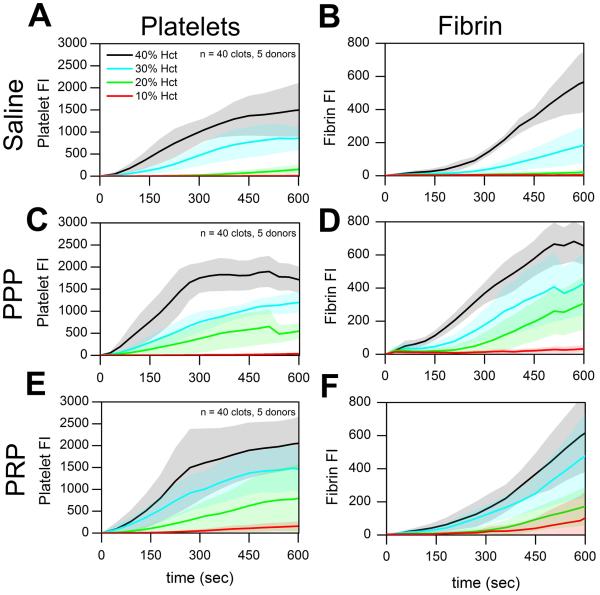
Similarly, in the presence of surface-triggered coagulation (Fig. 2), we detected significant decreases in platelet accumulation for all lowered Hct levels when whole blood was diluted with saline, PPP, or PRP (Fig. 2A, C, E; p < 0.05). Total fibrin accumulation at lower Hct levels was also significantly reduced when whole blood was supplemented with saline or PPP or PRP to reduce Hct (Figure 2B, D; p < 0.05). The reduced fibrin accumulation observed with reduced Hct cannot be due to a reduction in coagulation factors since the concentration of coagulation proteins remains constant when whole blood is diluted with PPP or PRP (as opposed to saline dilution). In this experiment, reduced platelet deposition was highly correlated with reduced fibrin production as Hct was reduced, regardless of how Hct was lowered (Figure 2).
Figure 2. Platelet and fibrin accumulation on TF-bearing collagen in healthy donors following Hct dilution.
(A & B), Platelet and fibrin fluorescence intensities vs. time with saline dilution of Hct. (C & D), Platelet and fibrin fluorescence intensities vs. time with PPP dilution of Hct. (E & F), Platelet and fibrin fluorescence intensities vs. time with PRP dilution of Hct. Shaded traces are the mean and standard deviation of 10 clotting events from 5 donors.
Exogenous tPA activates the lytic state at venous and arterial shear rates and promotes hyperfibrinolysis
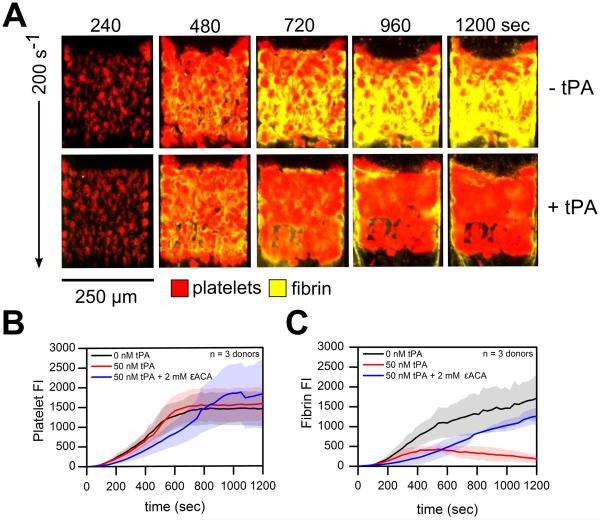
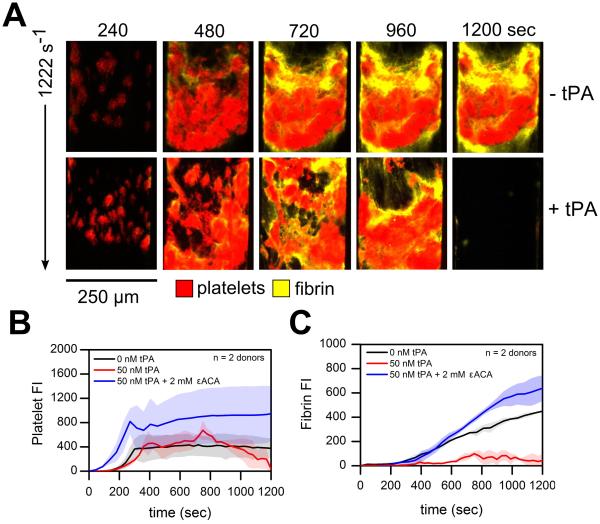
Following severe trauma and shock, injured endothelial cells release tPA systemically leading to hyperfibrinolysis (1). To mimic this pathological mechanism, tPA was exogenously added to whole blood (Supplemental Figure 1). At venous shear rates (200 s−1), exogenous addition of tPA to whole blood had negligible effects on platelet deposition on TF bearing collagen surfaces under flow (Figure 3A, B; ns). However, the rapid production of plasmin at the surface-patterned injury site in conjunction with fibrin formation at 200 s−1 induced a 'lytic state' with 50 nM ex vivo tPA addition. Total fibrin accumulation decreased 63.9 % by 10 min (Figure 3C; p <0.01). To reverse the 'lytic state' and stop fibrinolytic activity under flow, we added the lysine analogue ε-aminocaproic acid (εACA) to inhibit plasmin activity. The inhibition of plasmin by εACA restored total fibrin accumulation to levels comparable to control conditions but resulted in delayed fibrin initiation (Figure 4C). At arterial shear rates (1222s−1), embolization occurred with tPA as indicated by a drastic drop in the platelet signal by 12 min (Figure 4). The platelet signal continued to fall approaching zero by the end of the 20 min assay (Figure 4B). Exogenous tPA addition minimized total fibrin formation while treatment with εACA increased platelet deposition and total fibrin accumulation (Figure 4B, C; ns). Interestingly, in a few channels of the microfluidic assay at either venous or arterial wall shear rates, tPA-mediated fibrinolysis was followed by destabilization of platelet deposits which then embolized downstream. This disruption of platelet rich thrombi from the patterned injury site was followed a second round of platelet deposition and renewed fibrin generation (Supplemental Figure S4, S5)
Figure 3. Platelet and fibrin accumulation in response to exogenous tPA ± εACA at venous shear rates.
(A), Overlay of platelets (red) and fluorescent fibrinogen (yellow) deposition at 200 s−1 over the time course of the 20 min assay. (B), Platelet fluorescence intensities vs. time with exogenous tPA ± εACA. (C), Fibrin fluorescence intensities vs. time with exogenous tPA ± εACA. Shaded traces are the mean and standard deviation of 12 clotting events from 3 donors.
Figure 4. Platelet and fibrin accumulation in response to exogenous tPA ± εACA at arterial shear rates.
(A), Overlay of platelets (red) and fluorescent fibrinogen (yellow) deposition at 1222 s−1 over the time course of the 20 min assay. (B), Platelet fluorescence intensities vs. time with exogenous tPA ± εACA. (C), Fibrin fluorescence intensities vs. time with exogenous tPA ± εACA. Shaded traces are the mean and standard deviation of 8 clotting events from 2 donors.
Trauma patient platelet function tests: delayed platelet recruitment to collagen and attenuated secondary aggregation
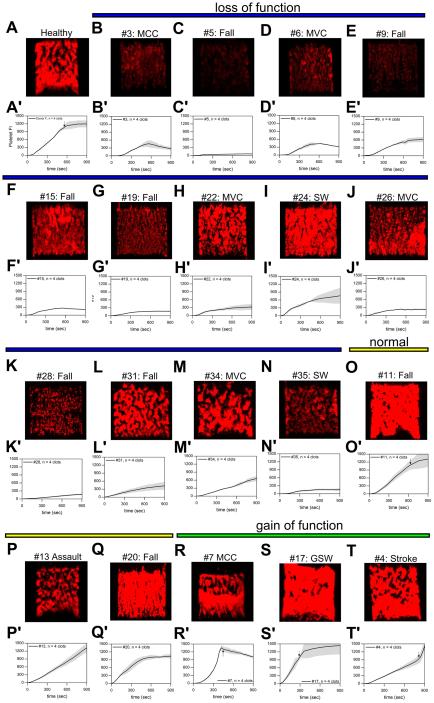
Rapid platelet function testing of PPACK-inhibited whole blood from trauma patients (n = 20) revealed a subpopulation of patients (14/20) with reduced platelet function upon arrival to a Level 1 Trauma Center (Figure 5, Supplemental Figure S8). When compared to 7 healthy donors, 14 trauma patients were found to have significantly decreased total platelet accumulation measured at 900 sec (Supplemental Figure S9; p < 0.01). We categorized these subset of patients as 'loss of function'. Patients with statically significant increased total platelet accumulation measured at 900 sec when compared to healthy donors were categorized as 'gain of function' (Figure 5, Supplemental Figure S9) Visual inspection of platelet aggregates formed on collagen surfaces indicate two loss of function phenotypes in trauma patients. The first loss of function phenotype in trauma patients was characterized by platelets failing to adhere to the collagen surface indicative of dysfunctional platelet glycoprotein VI (GPVI) (Figure 5B, C, E, K). In a second observed loss of function phenotype, platelet aggregate growth beyond the initial monolayer of platelets was minimal or completely missing (Figure 5D, F-N). Platelet aggregates tended to form above the initial monolayer of collagen-adherent platelets but were subsequently washed downstream. Clots formed from these patients did not grow to full channel occlusion during the duration of the 900 sec assay (Figure 5A, black arrow). This failure of secondary aggregation in these patients may be due to a lack of ADP or thromboxane A2 mediated clot growth. Interestingly, 3 patients exhibited hyper-responsive platelet function in this microfluidic assay with two patients forming occlusive clots within the first 400 seconds (Figure 5R-T).
Figure 5. Trauma patient thrombi morphology at 900 sec and platelet deposition dynamics at venous shear rates (100 s−1).
(A & A'), Platelet fluorescence intensities vs. time for representative healthy donor and representative image of final platelet accumulation at 900 sec. (B-N & B'-N', blue line), Platelet fluorescence intensities vs. time and representative image of final platelet accumulation at 900 sec for trauma patients exhibiting loss of platelet function. (O-Q & O'-Q', yellow line), Platelet fluorescence intensities vs. time and representative images of final platelet accumulation at 900 sec for trauma patients exhibiting normal platelet function. (R-T & R'-T', green line), Platelet fluorescence intensities vs. time and representative images of final platelet accumulation at 900 sec for trauma patients exhibiting gain of platelet function. Shaded traces are the mean and standard deviation of 4 clotting evens from each patient. (MCC: Motorcycle Crash, MVC: Motor Vehicle Crash, SW: Stab wound, GSW: Gun Shot Wound)
Trauma patient platelets respond less to antagonism by MRS 2179 and iloprost but increased sensitivity to inhibition by GSNO
To assess the various pathways that affect trauma platelet signaling under flow, we antagonized platelet function in three different manners (Supplemental Figure S2). MRS 2179 was used to potently inhibit the platelet ADP receptor, P2Y1. The addition of GSNO was used to stimulate nitric oxide production ex vivo and iloprost was used to raise cyclic adenosine monophosphate (cAMP) levels inside the platelet in order to reduce platelet aggregation. On average, trauma patients with detectable baseline platelet function measured by platelet fluorescence responded to all three forms of antagonism (Supplemental Figure S2C). Total platelet accumulation at 900 sec was reduced 58.8%, 43.83% and 73.80 % by MRS 2179, GSNO, and iloprost respectively in trauma patients (n = 17, 68 clots, p < 0.05) as compared to 79.2%, 15.2%, and 91.81% in healthy donors (n = 6, 24 clots, p < 0.05) (Supplemental Figure S9). Interestingly, trauma patient platelet function responded less to the addition of MRS 2179 or iloprost but was more strongly inhibited by production of nitric oxide ex vivo (Supplemental Figure S9; p < 0.01).
A subpopulation of trauma patient platelets displayed decreased p-selectin expression under flow
P-selectin surface expression was also used as a marker for α-granule secretion and irreversible platelet activation in our microfluidic assay (Supplemental Figure S3). A population of trauma patients (9/21) exhibited low p-selectin expression in the flow assay (data not shown) however, when normalized against the platelet fluorescence signal, a single patient displayed a severe deficit in p-selectin expression on a per platelet basis (Supplemental Figure S3B-D). The lack of p-selectin expression in patient #9 may be indicative of low levels of platelet activation and potential previous degranulation of platelets prior to microfluidic testing.
DISCUSSION
Trauma induced coagulopathy is a multi-faceted phenomenon that occurs in the combined setting of shock, hemodilution, hypothermia, and tissue injury. With the use of microfluidic technology we evaluated hemodilution and hyperfibrinolysis, two common mechanisms of TIC, in order to understand how derangements in platelet deposition and fibrin formation contribute to altered hemostasis. Hemodilution of healthy whole blood with saline, PPP, or PRP significantly reduced platelet adhesion to collagen in the absence of surface-triggered coagulation at all lowered Hct levels (Figure 1). Platelet deposition was also significantly decreased with this dilution scheme on TF-bearing collagen surfaces (Figure 2). RBCs strongly influences platelet margination and enhances platelet accumulation at the collagen or collagen/TF injury site in these assays. These results indicate a significant role for RBCs in mediating the rapid platelet response required to seal vessel injuries. Furthermore, RBCs have also been shown to release ADP thus promoting platelet aggregation and can potentially sustain thrombin generation (23). This is could be another role in which RBCs support platelet function and coagulation in our microfluidic assays. However, ADP release from RBC is expected to be minimal under the venous flow conditions tested (Figure 2).
Furthermore, a second mechanism associated with TIC is increased fibrinolytic activity. Excessive fibrinolysis impairs clot integrity and causes bleeding. We recapitulated this mechanism and rapidly detected changes in fibrin accumulation and clot stability in microfluidic assays with exogenous addition of tPA. We promoted a 'lytic state' under flow inducing fibrin lysis at venous shear rates that was rescued with ex vivo εACA addition (Figure 3). At arterial shear rates, the 'lytic state' induced embolism as clots tore from the TF bearing collagen surfaces and washed downstream (Figure 4). In rare cases, the 'lytic state' induced a consumptive coagulopathy with complete fibrin lysis and disintegration of platelet aggregates followed by platelet re-adherence and fibrin regeneration on the TF bearing collagen surface. This observed process depletes platelets, clotting factors, and plasma proteins in the flowing blood. The deranged hemostasis during hyperfibrinolysis we have recreated in the microfluidic assays mimics the coagulopathy of traumatic brain injuries (24). Our results suggest that hyperfibrinolysis and excessive consumption of clotting factors work synergistically during TIC. (Supplemental Figure S4, S5). Finally, we were able to detect and quantify changes in fibrin generation and clot stability during this 'lytic state' in <6 min, a time scale much faster than what is currently capable with TEG.
In clinical settings, conventional plasma-based assays are used to assess TIC instead of microfluidic-based assays. The prothrombin time (PT), partial thromboplastin time (PTT), platelet count, and fibrinogen levels are the most commonly used tests for monitoring coagulopathy following trauma. These tests, however, examine only a single component of the hemostatic process and fail to measure clot strength, fibrinolytic activity or platelet function. Furthermore, while TEG with or without platelet mapping is more indicative of global hemostasis, the use of FXII activator kaolin and citrated samples are major drawbacks with this technology. Kaolin activation of coagulation is non-physiologic as kaolin is not found in the body and citrate is known to affect platelet αIIβIIIa integrin function. The fluid mechanics of TEG also fail to replicate the hemodynamics of the vasculature. The oscillatory movement of a cup in a closed system cannot generate the extraordinary platelet concentrations in a clot on the vessel wall. Furthermore, in TEG, the moving cup is a closed system where platelet releasates and coagulation factors can accumulate unhindered due to the lack of flow-induced washout of these components. Microfluidic assessment of trauma patient function detected platelet function defects in < 5 min while a complete TEG test may take upward to 60 min (25).
In whole blood microfluidic assays, on the other hand, platelet deposition and fibrin generation must occur under well-controlled hemodynamic conditions and in the presence of convective dilution of thrombin, soluble agonists, and plasma proteins. During these assays, the biorhelogic phenomena present can either limit or augment local enzyme concentrations. In addition to changes in the local enzyme concentrations, platelet receptor-ligand bonds must withstand the hydrodynamic shear stress imparted by following blood. Thus whole blood microfluidic assays provide a much more rigorous physiologic test of platelet function and fibrin generation not captured by static clotting assays and TEG (26,27).
To the best of our knowledge, our results are the first real-time platelet function testing of whole blood samples from trauma patients using microfluidic technology. One previous study by Jacoby et al. has assessed platelet function following trauma using a platelet function analyzer (PFA-100) with citrated whole blood. The PFA-100 is a cartridge-based flow system that aspirates citrated whole blood in capillaries. It measures aperature closure time following blood clotting on a membrane coated with collagen/ADP or collagen/epinephrine. Their study reported decreased closure times at initial trauma patient presentation indicating increased platelet function and hypercoagulability. Contrary to the Jacoby et al. report, we observed noticeable decreases in platelet adhesion to collagen and secondary platelet aggregation when we rapidly assessed platelet function in trauma patients (n = 20) at 100s −1 over collagen surfaces (Figure 5). Furthermore, when comparing our microfluidic assay results to clinical patient data, we found no correlation between platelet function and injury severity score (ISS), or platelet function and the diagnosis of traumatic brain injury (TBI). Furthermore, all 14 patients displaying decreased platelet function had physiological hematocrit levels and platelet counts. With respect to static plasma clotting assays, 13 of the 14 patients with decreased platelet function were within the normal PTT reference range (20.8-34.4) (10). These results indicate that patient hematocrit levels and plasma-based clotting tests are largely inadequate in assessing the contribution of platelet function to acute traumatic coagulopathy.
Interestingly, of the 7 patients that did require blood products post microfluidic testing, 4 patients had decreased platelet function as initially assessed by whole blood microfluidic testing. A single patient (#31) was transfused with 14 units of blood products within 12 hrs of arrival. Microfluidic testing of this patient detected decreased platelet function upon initial presentation and continuous attenuated platelet function up to 72 hours post admission (Supplemental Figure S11).
While we were able to quickly measure and quantify trauma patient platelet dysfunction, the mechanisms underlying trauma platelet dysfunction require further investigation outside of microfluidic testing. Groups have postulated that platelet function downregulation can occur during trauma. Previous studies suggest the presence of dysfunctional circulating platelets following activation in response to tissue damage (5,7). Platelet receptor proteolysis may be another putative pathway in which platelet function is down-regulated in trauma patients. Previous reports describe proteolysis of platelet GPVI and GPIb-IX-V receptors (28,29). Ectodomain shedding of platelet adhesion receptors is a plausible physiologic mechanism in which platelets can respond to cases of severe endothelial injury following trauma yet prevent uncontrolled thrombus growth that leads to systemic clotting.
In this study, we have used microfluidic technology to effectively evaluate current resuscitation strategies and hyperfibrinolysis showing more rapid detection of impaired hemostasis and coagulation than what is capable with current technologies. Furthermore, we also tested trauma patient platelet function using this high throughput method. The fast identification of platelet function defects in trauma patients using whole blood microfluidic assays indicate a potential novel 15 min test to help guide coagulopathy treatments in the future.
Supplementary Material
ACKNOWLEDGMENTS
The authors stated that they had no interest which might be perceived as posing a conflict or bias.
This work was supported by the National Institutes of Health NIH R01 HL103419 (S.L.D.), NIH UMI HL120877 TACTIC Consortium.
Experiments were designed by R. Li, C. Sims, and S. L. Diamond. All experiments were performed by R. Li and H. Elmongy. The manuscript was written by all authors.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
REFERENCES
- 1.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65(4):748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 2.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet J-F. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway. Ann Surg. 2007;245(5):812–8. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, Allaouchiche B, Negrier C. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5(2):289–95. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 4.Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10(7):1342–51. doi: 10.1111/j.1538-7836.2012.04752.x. [DOI] [PubMed] [Google Scholar]
- 5.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73(1):13–9. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon C, Traintinger S, Ziegler B, Hanke A, Rahe-Meyer N, Voelckel W, Schochl H. Platelet function following trauma; A multiple electrode aggregometry study. Thromb Haemost. 2011;106(9):322–30. doi: 10.1160/TH11-03-0175. [DOI] [PubMed] [Google Scholar]
- 7.Jacoby RC, Owings JT, Holmes J, Battistella FD, Gosselin RC, Paglieroni TG. Platelet activation and function after trauma. J Trauma. 2001;51(4):639–47. doi: 10.1097/00005373-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CC, Ploplis V, Castellino FJ, Walsh M. Early platelet dysfunction: An unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214(5):739–46. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis PK, Musunuru H, Walsh M, Cassady R, Yount R, Losiniecki A, Moore EE, Wohlauer MV, Howard J, Ploplis VA, et al. Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit Care. 2013;18(2):201–8. doi: 10.1007/s12028-012-9745-6. [DOI] [PubMed] [Google Scholar]
- 10.Colace TV, Fogarty PF, Panckeri KA, Li R, Diamond SL. Microfluidic assay of hemophilic blood clotting: Distinct deficits in platelet and fibrin deposition at low factor levels. J Thromb Haemost. 2014;12(2):147–58. doi: 10.1111/jth.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maloney SF, Brass LF, Diamond SL. P2Y12 or P2Y1 inhibitors reduce platelet deposition in a microfluidic model of thrombosis while apyrase lacks efficacy under flow conditions. Integr Biol (Camb) 2010;2(4):183–92. doi: 10.1039/b919728a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colace TV, Muthard RW, Diamond SL. Thrombus growth and embolism on tissue factor-bearing collagen surfaces under flow: role of thrombin with and without fibrin. Arterioscler Thromb Vasc Biol. 2012;32(6):1466–76. doi: 10.1161/ATVBAHA.112.249789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rana K, Timmer BJ, Neeves KB. A combined microfluidic-microstencil method for patterning biomolecules and cells. Biomicrofluidics. 2014;8:056502. doi: 10.1063/1.4896231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Witt SM, Swieringa F, Cavill R, Lamers MME, van Kruchten R, Mastenbroek T, Baaten Constance, Coort S, Pugh N, Schulz A, et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat Commun. 2014;16(5):4257. doi: 10.1038/ncomms5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuijpers MJE, Van Der Meijden PEJ, Feijge MA, Mattheij NJ, May F, Govers-Riemslag J, Meijers JC, Heemskerk JW, Renne T, Cosemans JM. Factor XII regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler Thromb Vasc Biol. 2014;34(8):1674–80. doi: 10.1161/ATVBAHA.114.303315. [DOI] [PubMed] [Google Scholar]
- 16.Onasoga-Jarvis AA, Leiderman K, Fogelson AL, Wang M, Manco-Johnson MJ, Di Paola JA, Neeves KB. The Effect of Factor VIII Deficiencies and Replacement and Bypass Therapies on Thrombus Formation under Venous Flow Conditions in Microfluidic and Computational Models. PLoS One. 2013;8(11):e78732. doi: 10.1371/journal.pone.0078732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Panckeri KA, Fogarty PF, Diamond Recombinant factor VIIa enhances platelet deposition from flowing haemophilic blood but requires the contact pathway to promote fibrin deposition. Haemophilia. 2015;21(2):266–74. doi: 10.1111/hae.12558. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Fries S, Li R, Lawson JA, Propert KJ, Diamond SL, FitzGerald GA, Grosser T. Differential impairment of aspirin-dependent platelet cyclooxygenase acetylation by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci. 2014;111(47):16830–5. doi: 10.1073/pnas.1406997111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson PI, Stensballe J, Oliveri R, Wade CE, Ostrowski SR, Holcomb JB. How I treat patients with massive hemorrhage. Blood. 2014;124(20):3052–8. doi: 10.1182/blood-2014-05-575340. [DOI] [PubMed] [Google Scholar]
- 20.Bolliger D, Görlinger K, Tanaka KA. Pathophysiology and treatment of coagulopathy in massive hemorrhage and hemodilution. Anesthesiology. 2010;113(5):1205–19. doi: 10.1097/ALN.0b013e3181f22b5a. [DOI] [PubMed] [Google Scholar]
- 21.Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, Rosengart MR, Maier RV, Billiar TR, Peitzman AB. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986–93. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 22.Sihler KC, Napolitano LM. Massive transfusion: New insights. Chest. 2009;136(6):1654–67. doi: 10.1378/chest.09-0251. [DOI] [PubMed] [Google Scholar]
- 23.Neeves KB, Maloney SF, Fong KP, Schmaier AA, Kahn ML, Brass LF, Diamond SL. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. J Thromb Haemost. 2008;6(12):2193–201. doi: 10.1111/j.1538-7836.2008.03188.x. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee MS, Denney WS, Jing H, Diamond SL. Systems biology of coagulation initiation: kinetics of thrombin generation in resting and activated human blood. LoS Comput Biol. 2010;6(9):e1000950. doi: 10.1371/journal.pcbi.1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whelihan MF, Mann KG. The role of the red cell membrane in thrombin generation. Thromb Res. 2013;131(5):377–82. doi: 10.1016/j.thromres.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Stein SC, Smith DH. Coagulopathy in traumatic brain injury. Neurocrit Care. 2004;1(27):479–88. doi: 10.1385/NCC:1:4:479. [DOI] [PubMed] [Google Scholar]
- 27.Teodoro L, Nascimento B, Rizoli S. Thrombelastography (TEG®): practical considerations on its clinical use in trauma resuscitation. cand J Trauma Resusc Emerg Med. 2013;16(21):29. doi: 10.1186/1757-7241-21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neeves KB, Illing DA, Diamond SL. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys J. 2010;98(7):1344–52. doi: 10.1016/j.bpj.2009.12.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haynes LM, Dubief YC, Orfeo T, Mann KG. Dilutional control of prothrombin activation at physiologically relevant shear rates. Biophys J. 2011;100(3):765–73. doi: 10.1016/j.bpj.2010.12.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bender M, Hofmann S, Stegner D, Chalaris A, Bösl M, Braun A, Scheller J, Rose-John S, Niewandt B. Differentially regulated GPVI ectodomain shedding by multiple platelet expressed proteinases Differentially regulated GPVI ectodomain shedding by multiple platelet –expressed proteinases. Blood. 2010;116(17):3347–55. doi: 10.1182/blood-2010-06-289108. [DOI] [PubMed] [Google Scholar]
- 31.Bergmeier W, Piffath CL, Cheng G, Dole VS, Zhang Y, Von Andrian UH, Wagner DD. Tumor necrosis factor-α-converting enzyme (ADAM17) mediates GPIbα shedding from platelets in vitro and in vivo. Circ Res. 2004;95(7):677–83. doi: 10.1161/01.RES.0000143899.73453.11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.