Figure 4. The biosynthesis of MPT from cPMP.
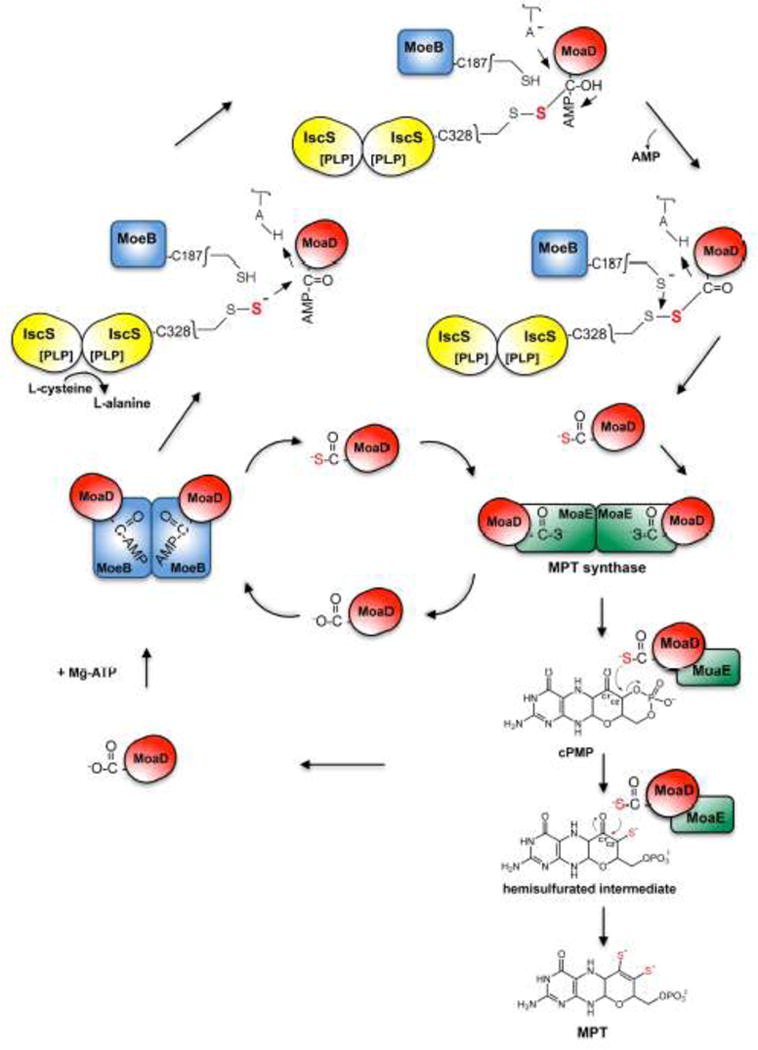
The MPT synthase tetramer is built of two MoaE and two MoaD subunits. In the MPT synthase mechanism, cPMP is bound to the MoaE subunit. The initial attack and transfer of the first thiocarboxylated MoaD-SH sulfur atom occurs at the C2′ position of cPMP, coupled to the hydrolysis of the cPMP cyclic phosphate. A new MoaD-SH thiocarboxylate attacks the C1′ of the newly formed hemisulfurated intermediate, which is converted to MPT via the elimination of a water molecule. MoaD is regenerated and a new MoaD-SH thiocarboxylate is formed on MoeB, where MoaD is first activated under ATP consumption to form an activated MoaD-acyl adenylate. MoaD-AMP is then sulfurated by a protein-bound persulfide, e.g. from Cys328 of IscS or from other sulfur transferring proteins like YnjE, TusA or SufS (not shown). Likely, an MoeB-MoaD disulfide intermediate is formed during the reaction, which is further cleaved by reductive cleavage (e.g. MoeB-Cys187). After formation of the thiocarboxylate group, MoaD-SH dissociates from the MoeB dimer and reassociates with MoaE, forming the active MPT synthase. The mechanism of MPT synthase was adapted from the one proposed in [14].
