Figure 5. Sulfur transfer to sulfur-containing biomolecules involving the IscS-bound persulfide.
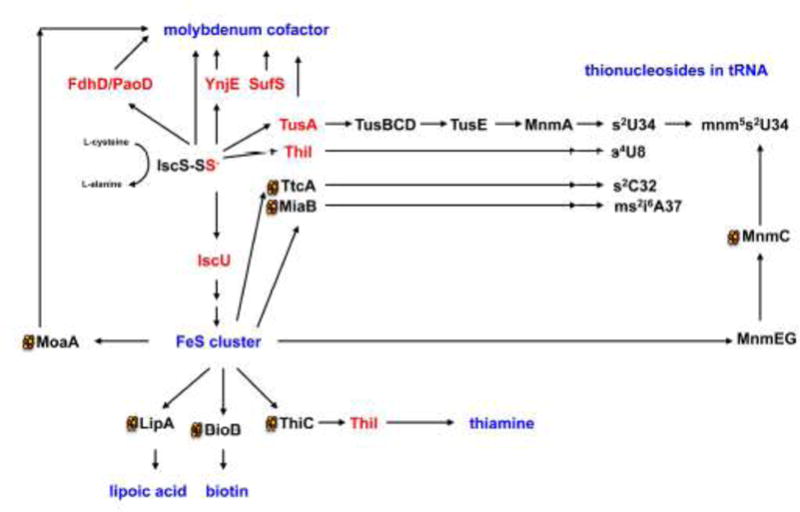
A protein-bound persulfide-group is formed on the L-cysteine desulfurase IscS from L-cysteine, releasing L-alanine. The persulfide-sulfur is further transferred to proteins like IscU, TusA, ThiI, YnjE, or FdhD (highlighted in red). IscU is the primary scaffold for the assembly of FeS clusters. FeS clusters are required for proteins involved in the pathways for the biosynthesis Moco, lipoic acid, biotin, thiamine and also for the addition of thionucleosides in tRNA. In E. coli tRNA thiolation involves the synthesis of s2C by TtcA, ms2i6A by MiaB, s4U by ThiI and a sulfur relay system involving TusA, TusBCD, TusE and MnmA for the formation of s2U in tRNA. In most cases, additional proteins are involved in the biosynthetic pathways. Persulfide-containing proteins are highlighted in red and names of the final sulfur-containing molecules are colored in blue. Proteins which containing 4Fe4S clusters are marked with a cluster.
