Figure 7.
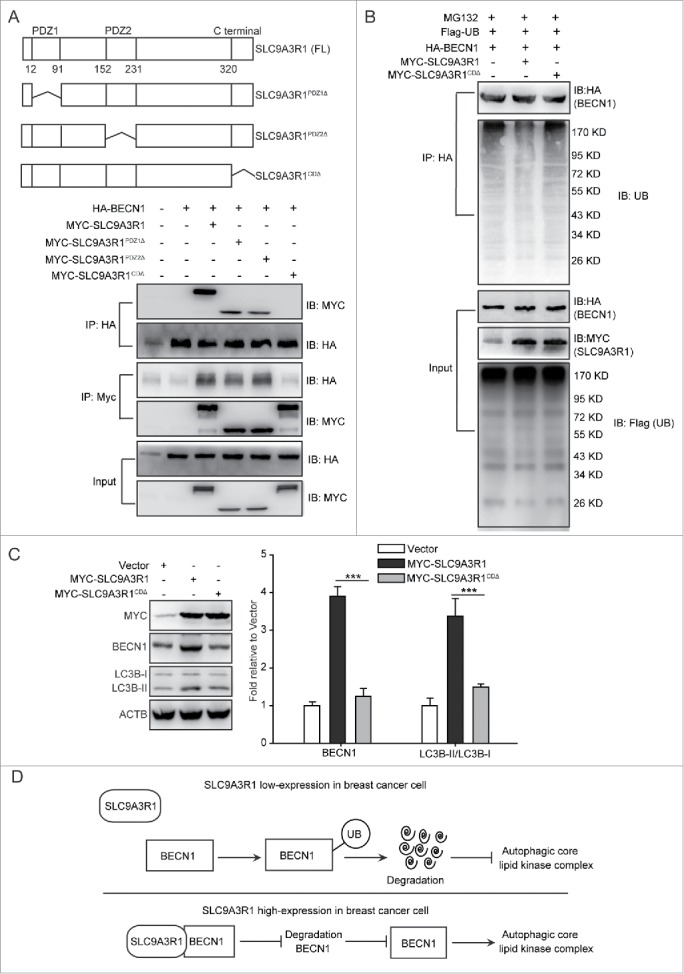
SLC9A3R1 stimulates autophagy by binding and stabilizing BECN1 in breast cancer cells. (A) Deficiency of the C-terminal domain of SLC9A3R1 inhibits the interaction between SLC9A3R1 and BECN1. Top: deletion mutants of SLC9A3R1. Below: MDA-MB-231 cells were cotransfected with HA-BECN1 and the indicated constructs of MYC-SLC9A3R1. Whole-cell extracts were immunoprecipitated with an anti-HA antibody. (B) Deficiency of the C-terminal domain of SLC9A3R1 does not affect the ubiquitination of BECN1. MDA-MB-231 cells were cotransfected with Flag-ubiquitin, HA-BECN1 and the indicated constructs of MYC-SLC9A3R1. The cells were then treated with MG132 (10 μmol/L) for 2 h. The cell lysates were extracted and immunoprecipitated using an anti-HA antibody. The precipitates were then analyzed with anti-ubiquitin and anti-HA antibodies. (C) Deficiency of the C-terminal domain of SLC9A3R1 does not induce autophagy. MDA-MB-231 cells stably expressing MYC-SLC9A3R1 or the indicated constructs of MYC-SLC9A3R1 were harvested and autophagy-related proteins were detected by western blot analysis. (D) Schematic diagram of the mechanism of SLC9A3R1-mediated autophagy stimulation in breast cancer cells. SLC9A3R1 interacts with BECN1 and inhibits the ubiquitin degradation of BECN1. Thus, enhanced BECN1 expression stimulates the autophagic core lipid kinase complex in breast cancer cells. Data are presented as the mean ± SE (n = 4). **, P < 0.01; ***, P < 0.001. UB, ubiquitin.
