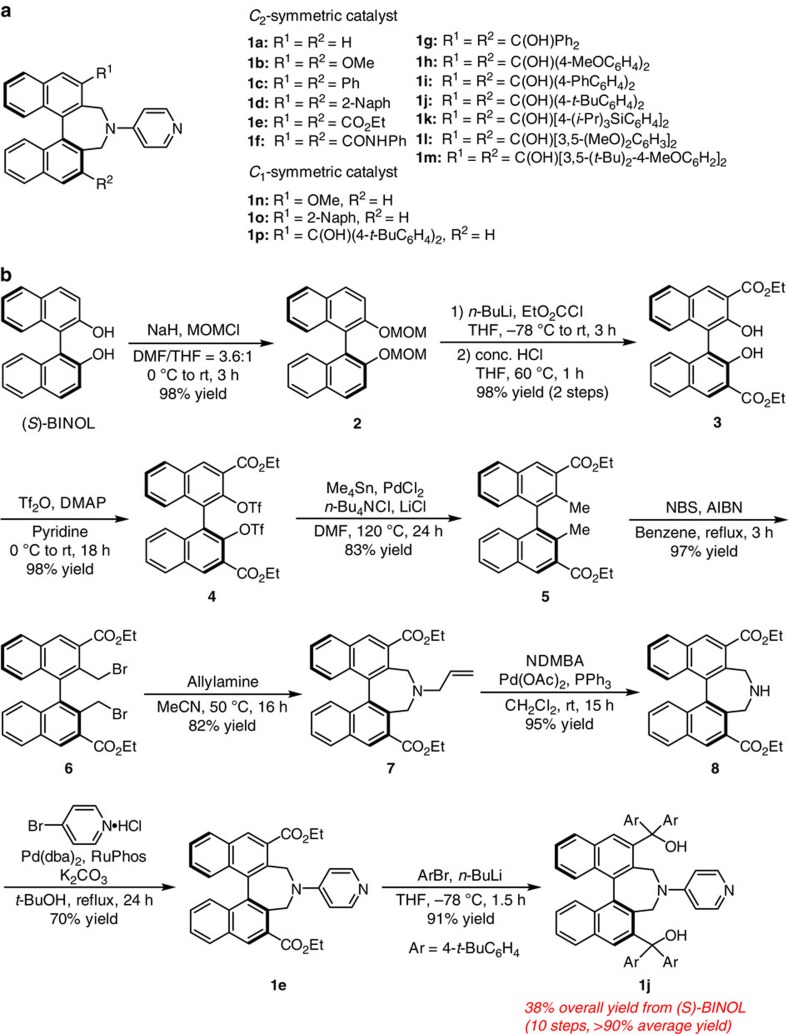
Figure 3. Synthesis of binaphthyl-based chiral nucleophilic catalyst candidates.
(a) Catalyst libraries containing C2- and C1-symmetic catalyst with different substitution pattern were used in the Steglich rearrangement of O-acylated oxindole derivatives. (b) C2-symmetric catalyst with polar functional group at 3,3′-positions of binaphthyl moiety can be prepared by a synthesis scheme that is high yielding and is readily amenable to scale-up with minimal column chromatography purification, as the representative example clearly illustrates. AIBN, 2,3′-azodiisobutyronitrile; NBS, N-bromosuccinimide; NDMBA, N,N′-dimethylbarbituric acid; RuPhos, 2-dicyclohexylphosphino-2′,6′-di-i-propoxy-1,1'-biphenyl.