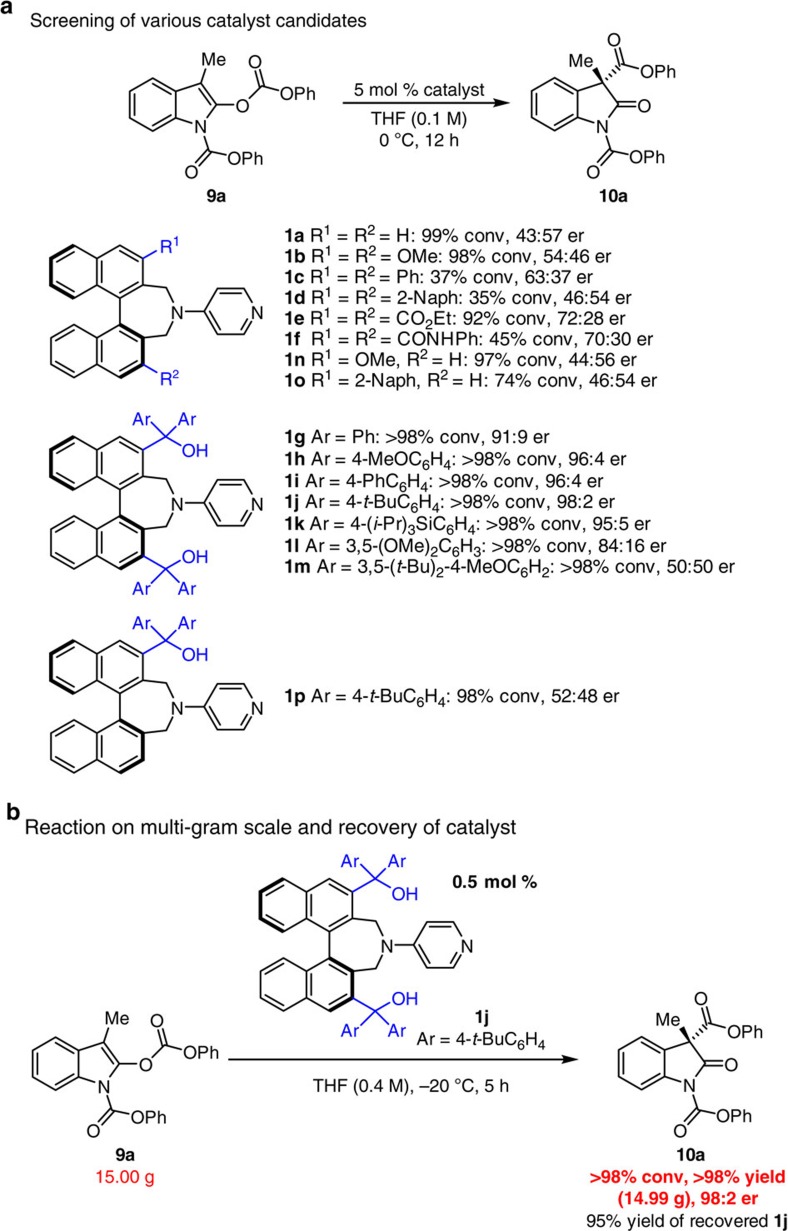
Figure 4. Enantioselective Steglich-type reactions promoted by catalyst 1j.
(a) Initial catalysts screening for the Steglich rearrangement of O-acylated oxindole derivative with C2- or C1-symmetric binaphthyl-based chiral nucleophilic catalyst. The results clearly indicated that tertiary alcohol unit at C3 and C3′ of binaphthyl are essential for achieving high yield and enantioselectivity. (b) The enantioselective reaction can be easily performed in multigram scale without any adverse effect on the efficiency or enantioselectivity of the process. Moreover, the same silica gel chromatography procedure delivers the recovered chiral catalyst in 95% yield.