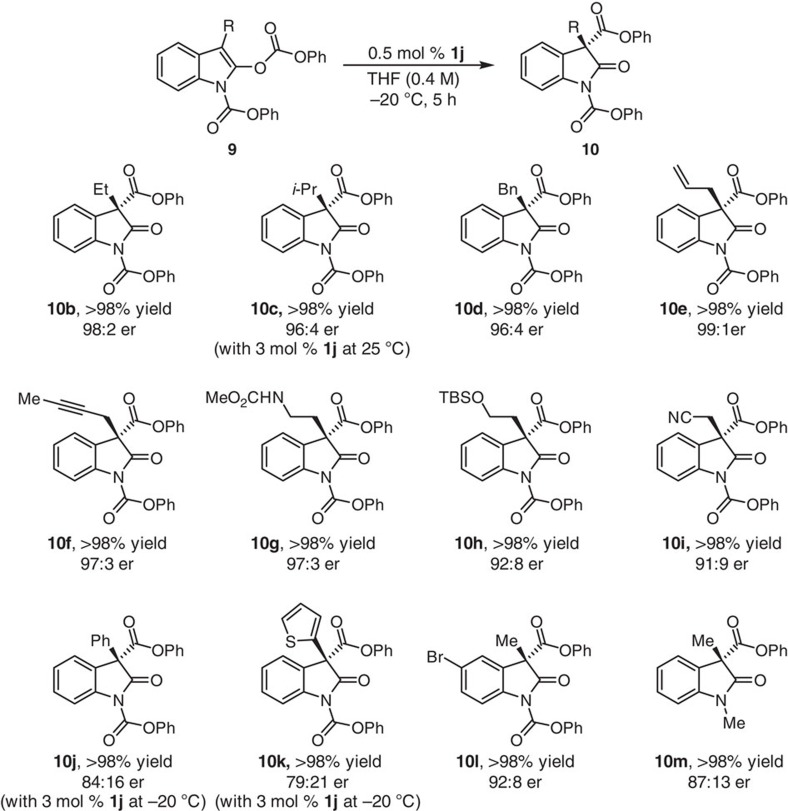
Figure 5. Enantioselective Steglich-type reactions of various substrates promoted by catalyst 1j.
A wide range of substrates may be subjected to enantioselective acyl rearrangement processes that afford quaternary carbon stereogenic centres in quantitative yield and in up to 98:2 er. The level of enantioselectivity can depend on the nature of the substituents within the oxindole ring. Reactions were performed on a 0.1 or 0.2 mmol (10b, 10d and 10l) scale in THF (0.4 M) under an argon atmosphere. Yields are of isolated and purified products after silica gel chromatography (±2%). Er values (±1%) were determined by high-performance liquid chromatography (HPLC) analysis.