Figure 5.
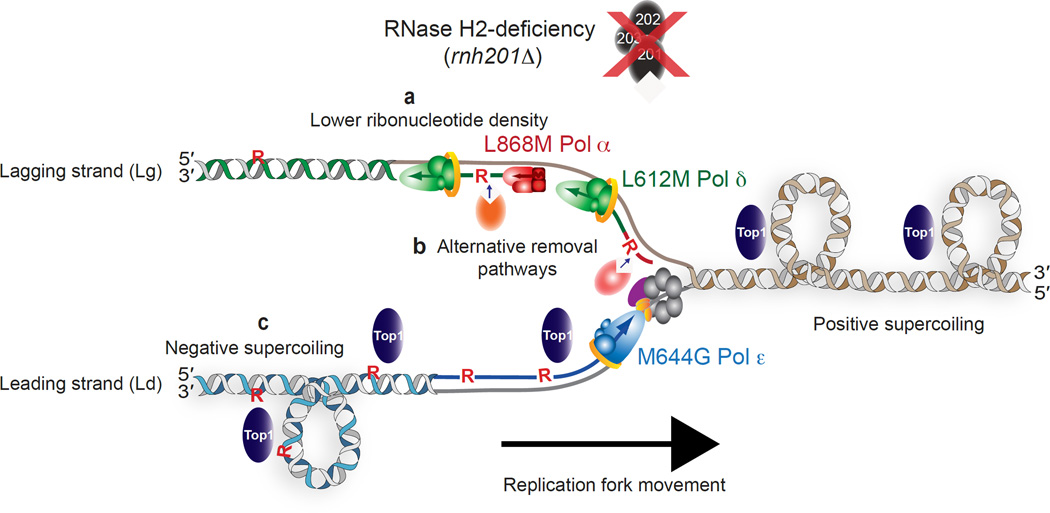
A model depicting three possibilities for strand-specific consequences of unrepaired ribonucleotides in the genomes of RER-defective yeast strains. (a) Failure to observe a Top1-dependent effect in the L868M Pol α or L612M Pol δ strains may be related to the fact that these enzymes incorporate fewer ribonucleotides into DNA than does the M644G Pol ε variant. This possibility is supported by the demonstration that deletion of RNH1 is lethal in the pol2-M644G rnh201Δ mutant but not in the pol1-L868M or pol3-L612M strains lacking RNH201 (Fig. 4d), a result that may be directly related to ribonucleotide density. Generation of alternative variants of Pol α and Pol δ that elevate ribonucleotide incorporation into nascent lagging strand DNA is an approach that could be taken to test this idea. (b) A second possibility is the existence of alternative ribonucleotide-repair pathways available on the lagging strand, perhaps involving enzymes involved in Okazaki fragment maturation, such as the Fen1 or Exo1 nucleases or the Dna2 helicase. (c) A third idea involves the possibility that negative supercoils accumulate in leading strand DNA in the wake of the replication fork. This type of superhelical tension may be related to the continuous nature of the leading strand, in contrast to the discontinuity of the lagging strand in the form of preexisting DNA nicks that may allow for DNA rotation and negate the need for relief of such supercoiling by the action of Top1. As a consequence, Top1 incision at ribonucleotides in the nascent leading strand then initiates the RNA-DNA damage observed in a pol2-M644G rnh201Δ mutant.