Figure 2.
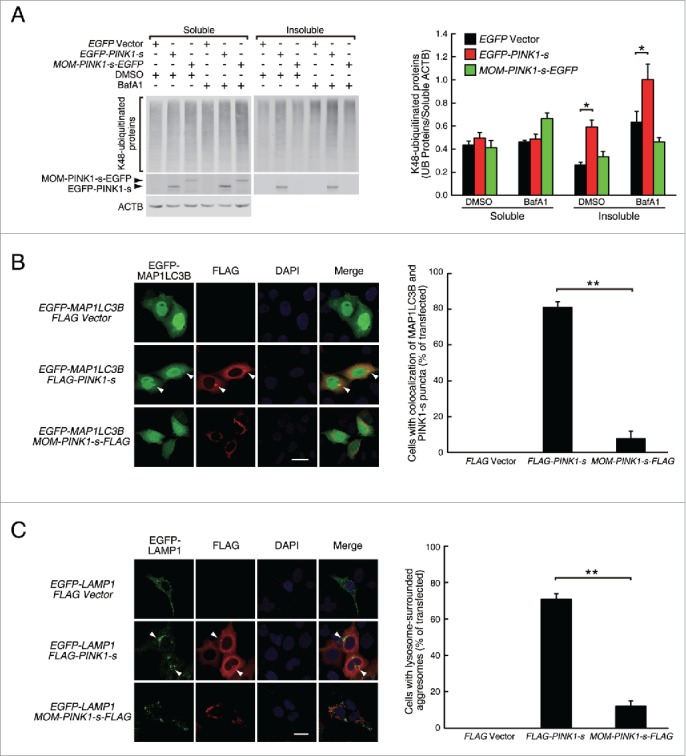
PINK1-s-induced aggresomes are targeted for autophagic degradation. (A) Western blot analysis of the soluble and insoluble K48-ubiquitinated proteins in AD293 cells transiently overexpressing EGFP, EGFP-PINK1-s or MOM-PINK1-s-EGFP. Twenty-four h after transfection, cells were treated with DMSO (control) or BafA1 (100 nM) for 12 h to inhibit the autophagic degradation of the aggregates and aggresomes. Sixteen percent of the soluble fraction and 50% of the insoluble fraction of each sample were loaded for SDS-PAGE and the levels of the ubiquitinated proteins and overexpressed PINK1 were detected by western blot analysis with UB(K48) and EGFP antibodies, respectively. Soluble ACTB (actin β) was detected and used as the loading control. Quantification results are shown as mean ± SEM of 3 independent experiments. *, P < 0.05. (B) Colocalization of EGFP-MAP1LC3 with PINK1-s-induced aggregate and aggresome. AD293 cells were transfected with the indicated plasmids for 24 h. FLAG-PINK1-s and MOM-PINK1-s-FLAG were detected by immunofluorescence staining with FLAG antibody. The nuclei were detected by DAPI staining. Scale bar: 20 μm. Quantification results are shown as mean ± SEM of 3 independent experiments. *, P < 0.05; NS, nonsignificant. (C) PINK1-s-induced aggresomes were surrounded by lysosomes. AD293 cells were transiently transfected with plasmids as indicated for 24 h. FLAG-PINK1-s and MOM-PINK1-s-FLAG were detected by immunofluorescence staining with FLAG antibody. The nuclei were detected by DAPI staining. Scale bar: 20 μm. Quantification results are shown as mean ± SEM of 3 independent experiments. **, P < 0.01.
