Figure 4.
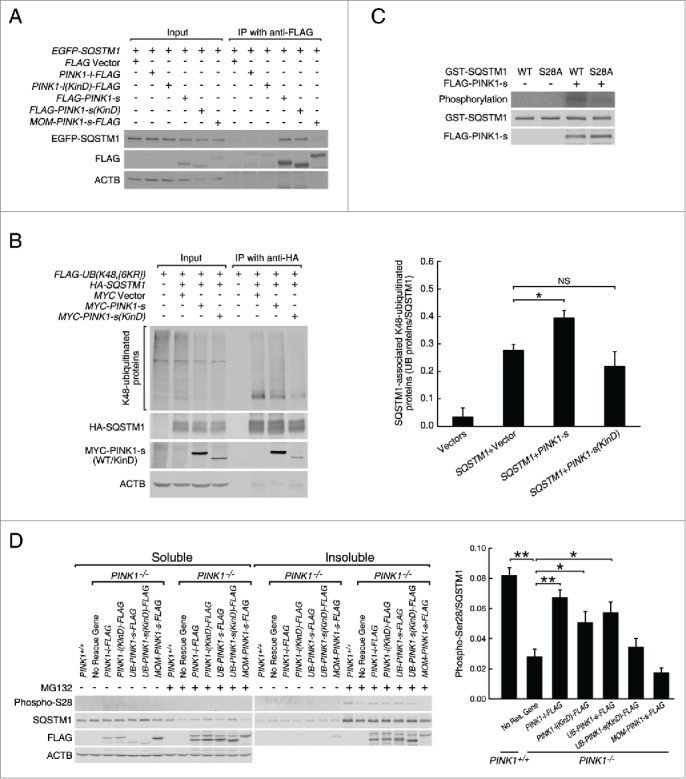
PINK1-s phosphorylates SQSTM1 at Ser28 during proteasomal stress. (A) Immunoprecipitation and western blot analysis of PINK1-SQSTM1 interaction. AD293 cells were transfected with the indicated plasmids. The FLAG-tagged PINK1 proteins were immunoprecipitated with FLAG antibody and the associated EGFP-SQSTM1 was detected with EGFP antibody. (B) Western blot analysis of SQSTM1-bound, K48-ubiquitinated proteins in control cells and cells expressing MYC-PINK1-s or MYC-PINK1-s(KinD). Cells were transfected with the indicated plasmids and immunoprecipitated with HA antibody. HA-SQSTM1-bound ubiquitinated proteins were detected with FLAG antibody. Quantification results are shown as mean±SEM of 3 independent experiments. *, P < 0.05; NS, non-significant. (C) In vitro phosphorylation assay of the wild-type (WT) and S28A mutant GST-SQSTM1 by PINK1-s. GST-SQSTM1(WT) and GST-SQSTM1S28A were expressed in bacteria and affinity purified with glutathione beads. FLAG-PINK1-s was expressed in AD293 cells and immunopurified with FLAG antibody. GST-SQSTM1(WT) or GST-SQSTM1S28A was incubated with FLAG-PINK1-s in the presence of γ-32P-ATP to label the phosphorylated proteins. GST-SQSTM1 and FLAG-PINK1-s in the reactions were detected by western blot analysis with GST and FLAG antibodies, respectively. (D) Western blot analysis of SQSTM1 Ser28 phosphorylation in the soluble and insoluble fractions of PINK1+/+, PINK1−/− and rescue cells after a 12-h treatment with DMSO or MG132 (1 μM). Eight percent of the soluble and 20% of the insoluble fractions of each sample were loaded for SDS-PAGE and western blot analysis. Ser28 phosphorylation was detected with a custom-made antibody. Soluble ACTB levels were detected as the loading control. Quantification results are shown as mean±SEM of 3 independent experiments. *, P < 0.05; **, P < 0.01.
