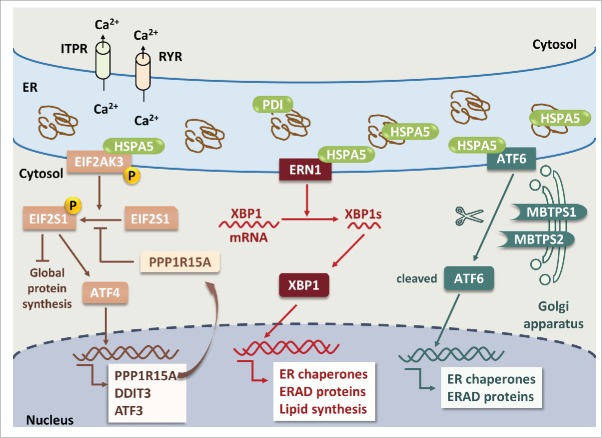
Figure 1.
ER stress and UPR pathways in neuronal cells. Pathological accumulation of misfolded proteins and/or depletion of ER calcium store via activation of ITPR and RYR leads to ER stress. Dissociation of HSPA5 from 3 ER stress sensors, EIF2AK3, ERN1 and ATF6, results in phosphorylation of EIF2AK3 and ERN1 and translocation of ATF6 to the Golgi apparatus. Activated EIF2AK3 is a serine/threonine protein kinase that phosphorylates EIF2S1. p-EIF2S1 (phosphorylated EIF2S1) inhibits global protein synthesis but selectively upregulates ATF4, PPP1R15A, DDIT3 and ATF3. PPP1R15A provides a negative feedback by dephosphorylating p-EIF2S1. ERN1 cleaves XBP1 mRNA; the spliced form of XBP1 encodes the XBP1 protein. XBP1 increases the expression of genes encoding ER chaperones, ERAD proteins and lipid synthesis to restore the capacity of protein folding. ATF6 is translocated to the Golgi apparatus where it is cleaved by MBTPS1 and MBTPS2, and cleaved ATF6 stimulates the expression of ER chaperones and ERAD proteins. Apoptosis will ensue if upregulation of ER chaperones and ERAD proteins fails to rescue ER stress.