Figure 6.
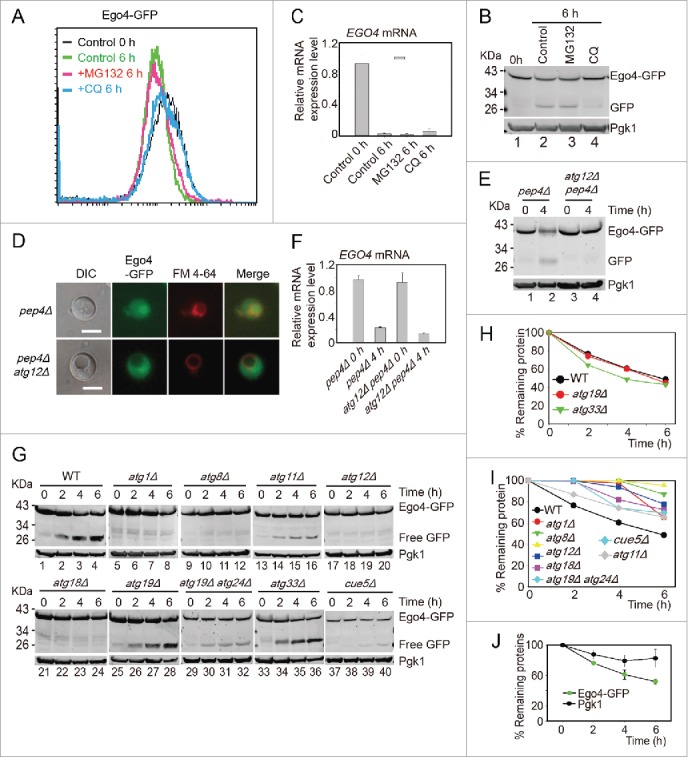
Ego4 is selectively degraded by autophagy. (A-C) The degradation of Ego4 was not dependent on the proteasome but dependent on autophagy. The EGO4-GFP strain was transferred into SPM and treated with or without 100 μM MG132 or 200 μM CQ between 0 to 6 h. The GFP intensity and EGO4 mRNA expression level were analyzed by flow cytometry (A), western blotting (B) and quantitative real time PCR (C). (D-F) Ego4-GFP was transferred into the vacuole at early stage of meiosis, which was dependent on autophagy. pep4Δ or pep4Δ atg12Δ cells were prepared as in Fig. 1A. The 4-h samples from SPM were visualized by fluorescence microscopy (D) and protein changes were detected by western blotting (E). Scale bar: 5 μm. DIC, differential interference contrast. The mRNA expression levels of those samples were analyzed by real-time PCR (F). (G) Ego4-GFP was selectively degraded by autophagy. GFP-tagged Ego4 proteins in WT, atg1Δ, atg8Δ, atg12Δ, atg18Δ, atg19Δ, atg33Δ, atg11Δ, atg19Δ atg24Δ and cue5Δ strains were analyzed by western blotting (lanes 1-40). Samples were collected at 0, 2, 4 and 6 h after transferring into SPM. (H-I) Quantitative analysis of Ego4-GFP degradation in ATG knockout strains in (G). The degradation rates of Ego4-GFP in WT, atg19Δ, atg33Δ (H) atg1Δ, atg8Δ, atg11Δ, atg12Δ, atg18Δ, atg19Δ atg24Δ and cue5Δ (I) strains. (J) Degradation of Ego4-GFP was faster than Pgk1. The EGO4-GFP strain was sporulated and samples were collected at 0, 2, 4, and 6 h for western blotting. The percentages of remaining protein were the value of the protein level divided by their protein levels at 0 h.
