Figure 21.
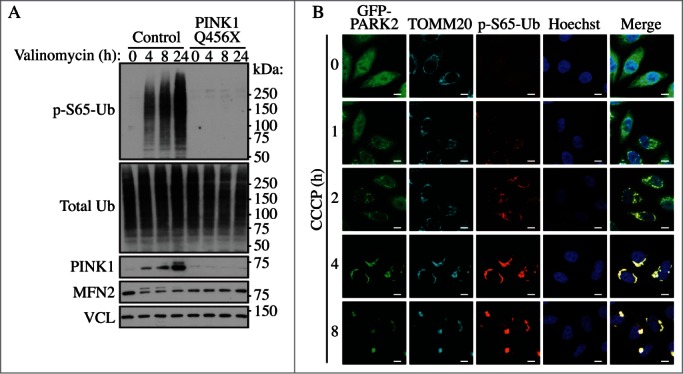
PINK1-dependent phosphorylation of ubiquitin (p-S65-Ub) upon mitophagic stress. (A) Human dermal fibroblasts from healthy controls or Parkinson disease patients carrying a PINK1 loss-of-function mutation (Q456X) were treated with valinomycin for the indicated times and lysates were analyzed by western blot. The p-S65-Ub signal is almost undetectable under nonstress conditions in controls, but is strongly induced in a PINK1 kinase-dependent manner during its stabilization on the outer mitochondrial membrane. MFN2 serves as a control substrate and VCL (vinculin) as a loading control. (B) HeLa cells stably expressing GFP-PARK2 (wild type) were treated with CCCP for the indicated times, fixed and stained with p-S65-Ub (red) and GFP-PARK2 (green) as well as mitochondrial (TOMM20, cyan) and nuclear (Hoechst, blue) markers. The p-S65-Ub staining is almost undetectable in nonstressed cells, but rapidly accumulates on damaged mitochondria where it functions to activate PARK2. On mitochondria, PINK1 and PARK2 together amplify the p-S65-Ub signal. Scale bar: 10 µm. Image provided by F.C. Fiesel and W. Springer.
