Figure 2.
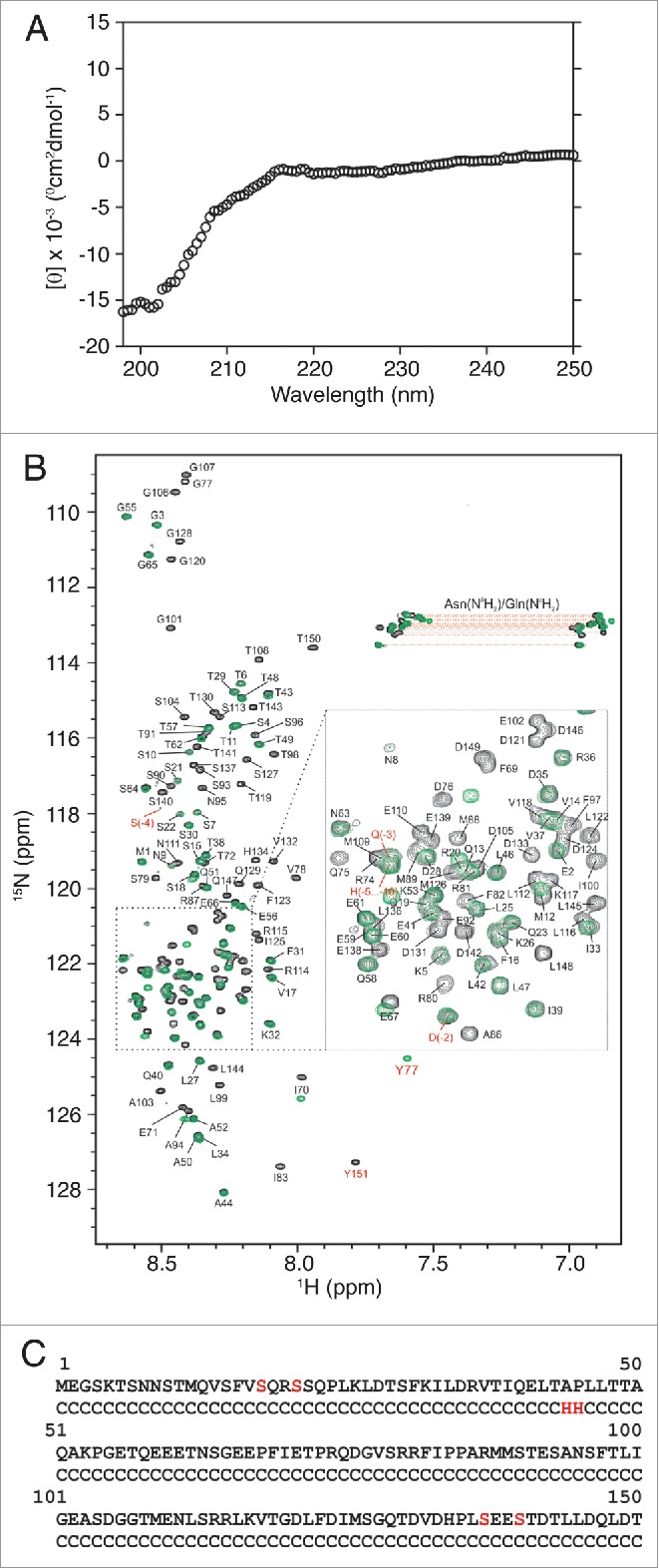
The BECN1 N-terminal domain is intrinsically disordered. (A) Circular dichroism spectra of BECN1(1–150)[4CS]Y. The mean residue ellipticity ([θ]) is plotted as a function of wavelength. (B) Overlay of 1H-15N HSQC spectra of BECN1(1–150)[4CS]Y (black) and BECN1(1-76)[2CS]Y (green) with backbone assignments of BECN1(1–150)[4CS]Y annotated. Resonances corresponding to side-chain NH2 amides of Asn and Gln are connected by horizontal lines. Resonances arising from the short linker sequence at the N terminus and the extra tyrosine residue added to the C terminus are labeled in red. (C) Secondary structure of BECN1(1–150)[4CS]Y as predicted by CSI 2.034 based on experimentally observed backbone chemical shifts (13Cα, 13Cβ, 13C, 15N, 1HN, and 1Hα). C: random coil, H: helix. Serine substitutions for native cysteines are indicated in red.
