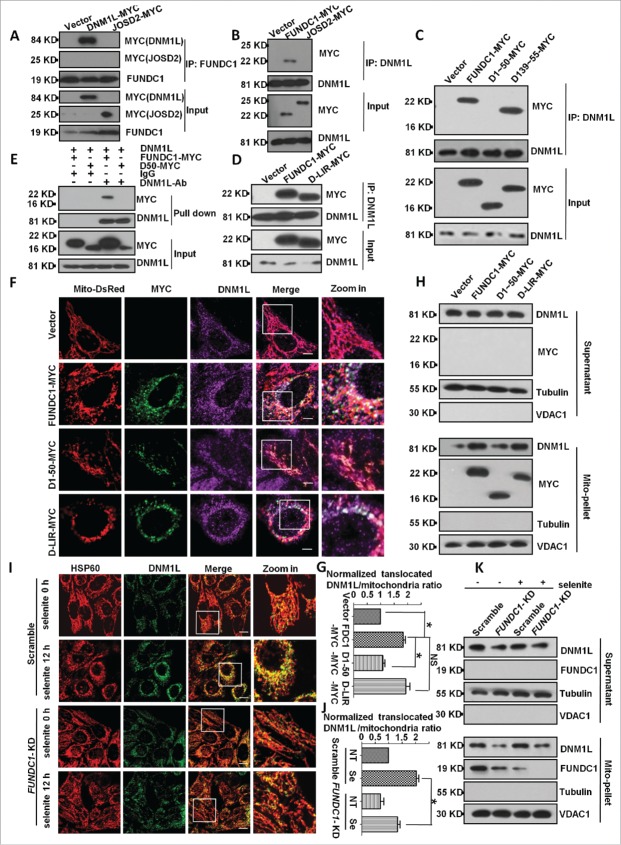
Figure 2.
FUNDC1 interacts with, and recruits DNM1L to mitochondria. (A and B) HeLa cells were transfected with FUNDC1-MYC or DNM1L-MYC (JOSD2-MYC was used as negative control) for 24 h, harvested and lysed, and they were then subjected to immunoprecipitation (IP) with an anti-DNM1L antibody (A) or anti-FUNDC1 antibody (B). CoIP with FUNDC1 (A) or DNM1L (B) was detected by western blotting using an anti-MYC antibody. ((C)and D) HeLa cells were transfected with FUNDC1-MYC or the indicated mutants for 24 h and then were subjected to immunoprecipitation (IP) with an anti-DNM1L antibody. wild-type FUNDC1 and FUNDC1 mutants were detected through western blotting using an anti-MYC antibody. (E) 0.5 μg DNM1L protein was incubated with 1 μg anti-DNM1L antibody and 30 μl protein G-Sepharose beads in 500 μl 1% NP-40 lysis buffer (pH 7.4) following incubating for 4 h and washing 3 times with PBS. HeLa cells were transfected with FUNDC1-MYC or the indicated mutant for 24 h and then were lysed in 1% NP-40 lysis buffer for 30 min. Cell lysis and protein G-Sepharose beads were incubated for 6 h following washing 5 times. The affinity isolation of FUNDC1-MYC (wild type or its mutant) was detected by western blotting using an anti-MYC-antibody. (F) HeLa cells were transfected with Mito-DsRed and FUNDC1 or its mutants for 24 h. The cells were then fixed and immunostained to detect MYC (green) and DNM1L (purple). Scale bar: 10 µm. (G) The proportion of mitochondria translocated DNM1L in (F) were quantified with imageJ by measuring DNM1L (purple) mitochondria (red) merged area and mitochondria area (red), merged area vs. mitochondria area was used to indicate the translocated DNM1L. The ratio was normalized to cells transfected with scrambled shRNA vector plasmids (mean+/−SEM; n = 100 cells from 3 independent experiments; *, P < 0 .05). (H) HeLa cells were subfractionated to detect DNM1L translocation from the cytosol fraction to mitochondrial pellets in the transfection in (F). (I) Scrambled shRNA-treated and FUNDC1 knockdown cells were then treated with 10 µM selenite for 12 h and then fixed and immunostained to detect DNM1L (green) and HSP60 (red). Scale bar: 10 µm. (J) The proportion of mitochondria-translocated DNM1L in (I) was quantified with imageJ by measuring DNM1L (green) mitochondria (red) merged area (yellow) and mitochondria area (red), the ratio of translocated DNM1L was analyzed as in (G) (mean+/−SEM; n = 100 cells from 3 independent experiments; *, P < 0 .05). (K) HeLa cells were subfractionated to detect DNM1L translocation from the cytosol fraction to mitochondrial pellets in the treatment in (I).