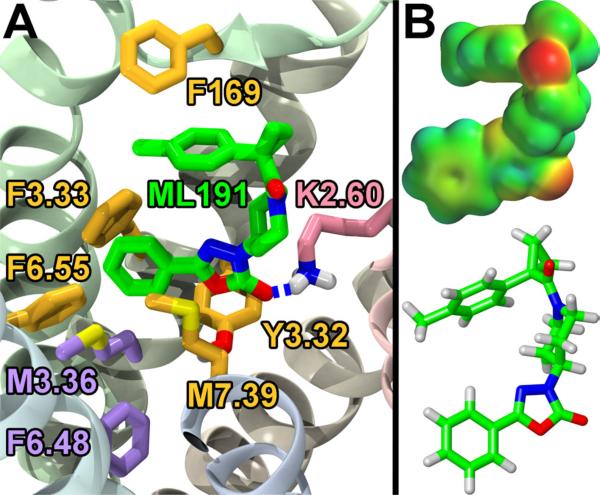
Figure 2.
A. Docking and Key Interactions Between ML191 and GPR55. ML191 (green) has a key H-bond interaction with K2.60 (pink). ML191 also has π-stacking or other van der Waals inter-actions with F169, F3.33, F6.55, M7.39, and Y3.32 (all mustard). The interactions with M7.39 and F6.55 appear to hinder the rotation of M3.36 and F6.48 (both purple) which are considered the toggle switch for GPR55. B. Electrostatic potential map of ML191. [This figure is adapted from previously published work, see ref. 12].