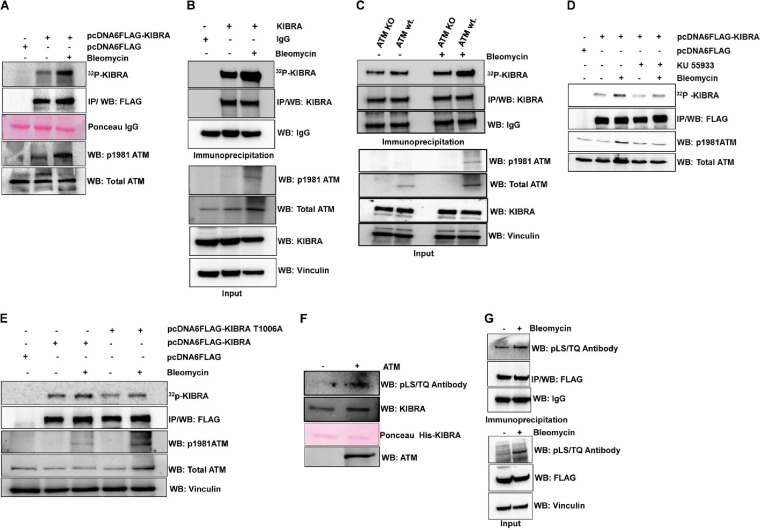
FIG 2.
ATM kinase phosphorylates KIBRA in vivo at threonine 1006 site. (A) HEK293T cells were transfected with pcDNA6-FLAG and pcDNA6-FLAG-KIBRA (wt.) plasmids. After 24 h, transfected cells were metabolically labeled with [32P]orthophosphoric acid overnight, followed by treatment with bleomycin for 2 h, FLAG immunoprecipitation (IP) from cell lysates, and gel electrophoresis. Phosphorylated protein bands were analyzed with autoradiogram images and subsequent immune blotting with anti-FLAG antibody, anti-phosphor-ATM, and anti-total ATM antibody, and the Ponceau image shows that equal amounts of FLAG beads were used for IP. (B) Endogenous metabolic labeling was performed in MDA-MB-231 cells. Cells were metabolically labeled with [32P]orthophosphoric acid overnight, followed by treatment with bleomycin for 2 h and then KIBRA IP from cell lysates and gel electrophoresis. Phosphorylated protein bands were analyzed with autoradiogram images and subsequent immune blotting with anti-KIBRA antibody. Input lysates were probed with anti-KIBRA, ATM antivinculin as a loading control, and anti-phospho-ATM antibody for confirming ATM activation. (C) ATM KO (GM16666) and ATM wt. (GM16667) cells were metabolically labeled with [32P]orthophosphoric acid overnight, followed by treatment with bleomycin for 2 h, and then KIBRA was immunoprecipitated from cell lysates. Phosphorylated protein bands were analyzed with autoradiogram images and subsequent immune blotting with anti-KIBRA antibody. Input lysates were probed with anti-KIBRA, anti-ATM, antivinculin, and anti-phospho-ATM antibodies. (D) HEK293T cells were transfected with pcDNA6-FLAG and pcDNA6-FLAG-KIBRA-wt. plasmids. After 24 h, transfected cells were metabolically labeled with [32P]orthophosphoric acid overnight; cells were then treated with KU-55933 (10 μmol/liter) for 3 h and with bleomycin for 2 h, and then FLAG immunoprecipitation (IP) from cell lysates and gel electrophoresis was performed. Phosphorylated protein bands were analyzed with autoradiogram images and subsequent immune blotting with anti-FLAG antibody, anti-phospho-ATM, and anti-total ATM antibody. (E) HEK293T cells were transfected with pcDNA6-FLAG, pcDNA6-FLAG-KIBRA-wt., and pcDNA6-FLAG-KIBRA–T1006A plasmids. After 24 h, transfected cells were metabolically labeled with [32P]orthophosphoric acid overnight; cells were then treated with bleomycin for 2 h, and then FLAG immunoprecipitation (IP) from cell lysates and gel electrophoresis was performed. Phosphorylated protein bands were analyzed with autoradiogram images and subsequent immune blotting with anti-FLAG antibody, anti-phospho-ATM, and anti-total ATM antibody. (F) In vitro kinase assay with His-KIBRA (aa 1119) in the presence or absence of ATM kinase. The blot was probed with the phospho-(Ser/Thr) ATM/ATR substrate antibody (S/T) (Q motif-specific) antibody and subsequently with anti-KIBRA and anti-ATM, and the Ponceau image shows equal loading of the protein. (G) HEK293T cells were transfected with pcDNA6-FLAG-KIBRA (wt.) plasmid; after 48 h, cells were treated with or without bleomycin for 2 h followed by FLAG immunoprecipitation from cell lysates and gel electrophoresis, and the blot was probed with the indicated antibodies.