FIG 7.
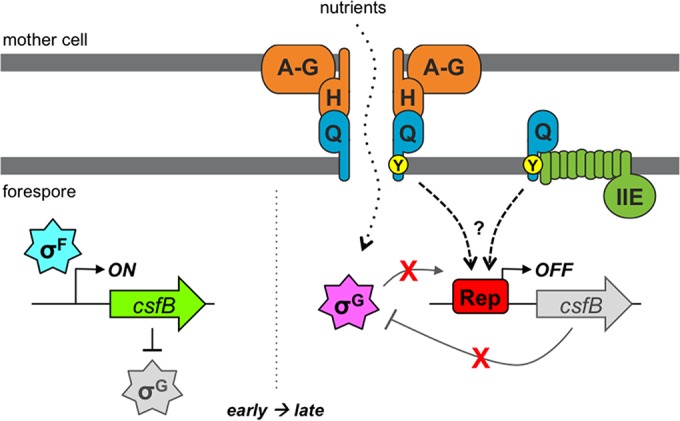
A model for the Q Tyr-28-mediated csfB regulatory pathway. After asymmetric division and during engulfment (early, left), transcription of the csfB gene is activated by σF in the forespore. In turn, the produced CsfB protein acts as an inhibitor of σG, helping to prevent its premature activation. After the completion of forespore engulfment (late, right), the transcription of csfB by σG is prevented by a conserved csfB promoter element, which we propose is a binding site for a repressor protein (Rep). The cessation of csfB transcription limits CsfB production to promote maximal σG activation. A highly conserved tyrosine (Tyr-28; Y) in the TMD of Q (whose gene is also activated by σF [not shown]) is also required for the inhibition of σG-dependent csfB transcription. Q is known to assemble into a channel apparatus with the mother cell proteins AA-AH (A-H) and to form a direct interaction with AH (H). This channel is generally required for gene expression at late times in the forespore, including that directed by σG; as such, it has been proposed to serve as a feeding tube through which the mother cell provides nutrients required for macromolecular synthesis (as shown). A separate subpopulation of Q is also known to interact with the SpoIIE phosphatase (IIE), an interaction that we have shown in the current study to be dependent specifically on Q Tyr-28. We propose that this subpopulation of Q, in collaboration with SpoIIE, positively regulates the csfB repressor protein by an unknown mechanism. However, we cannot rule out other scenarios, including the possibility that SpoIIE is not involved and/or that Q interacts with yet another partner via Tyr-28 to effect csfB regulation. Regardless, our data support the conclusion that Q is a bifunctional protein that (i) generally activates σG, through its assembly into the AA-AH·Q channel, and (ii) specifically maximizes the activity of σG, through participation in a gene regulatory circuit that represses expression of the gene encoding the anti-σG factor CsfB.
