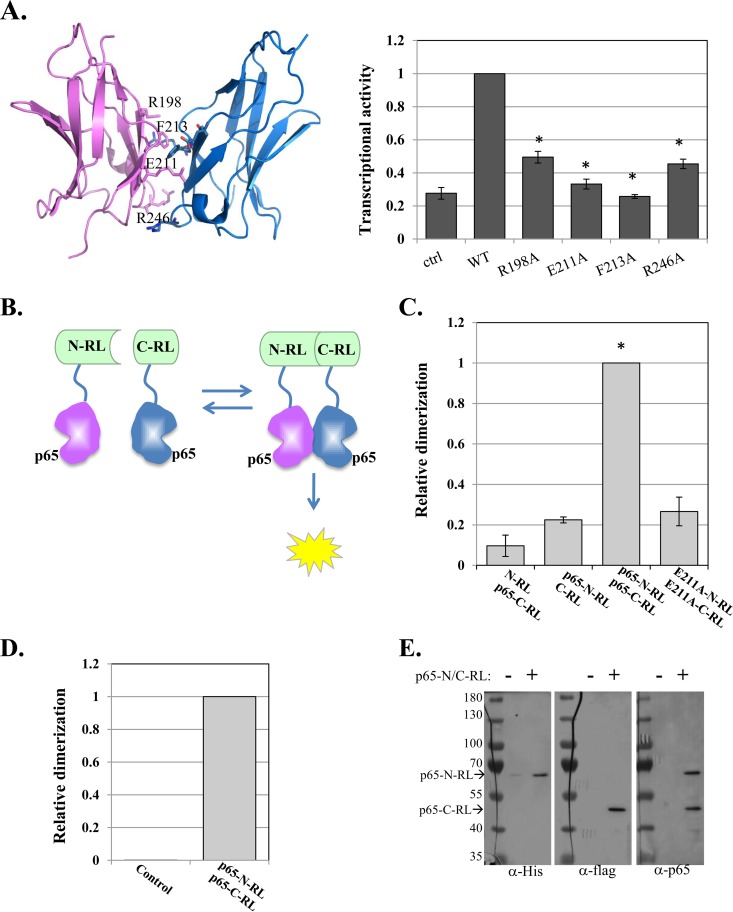
FIG 1.
Design and application of a high-throughput screen for direct inhibitors of p65 dimerization. (A) (Left) p65-p65 homodimer structure (aa 191 to 304) (PDB ID 1MY5) (14) with dimerization interface residues labeled. (Right) WT p65 and dimerization interface mutants were transfected into cells, together with the NF-κB target reporter A20-luciferase and RSV-Renilla luciferase, which served as a control for transfection efficiency. Twenty-four hours after transfection, the cells were harvested and luciferase activities were measured. The bars represent the means and standard errors (SE) of the results of 3 independent experiments. (B) Schematic illustration of the p65–split-Renilla luciferase principle. (C) The N and C termini of the RL fused to p65 were transfected into cells, together with miR-22–FL, which served as an internal control. RL and FL activities were measured after 24 h. The bars represent the means ± standard errors of the mean (SEM) of the results of 3 independent experiments. The asterisks denote statistically significance differences (P < 0.05). The activity of the WT p65-C/N-RL pair was set to 1. (D) E. coli was transformed either with an empty plasmid or with a plasmid directing expression of p65-N/C-RL fusion proteins. Following induction, the cell lysates were analyzed for RL enzymatic activity. (E) The p65-N/C-RL proteins were analyzed by Western blotting with the antibodies indicated at the bottom.