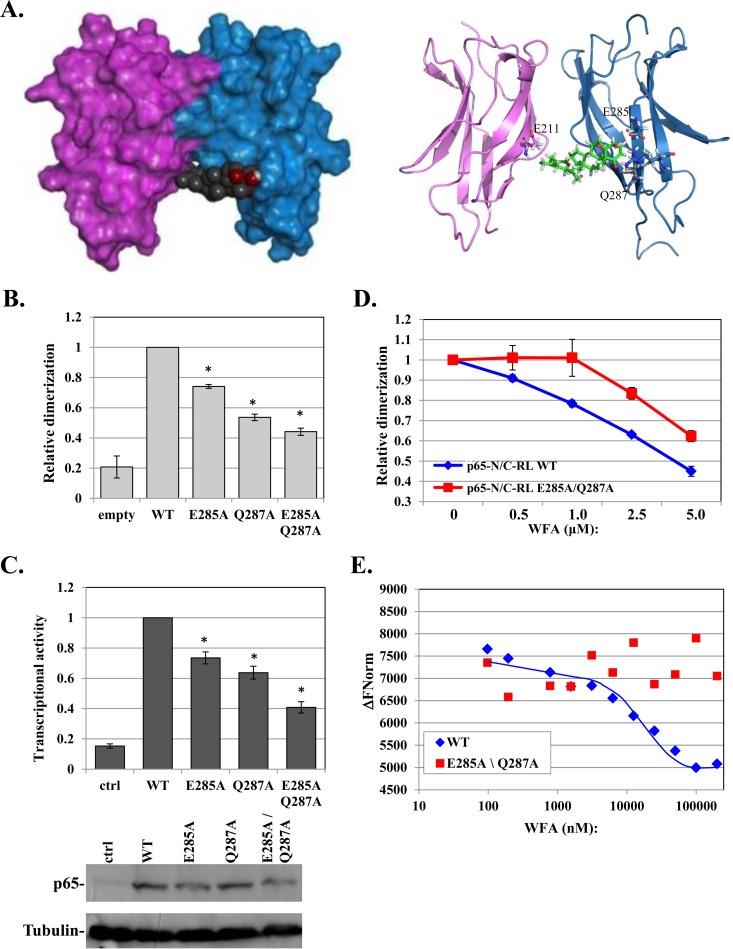
FIG 4.
WFA-bound p65 revealed E285 and Q287 as allosteric modulators of p65 dimerization. (A) Computational models of a p65-p65 homodimer (PDB ID 1MY5) (14) in a complex with WFA using the Schrödinger program. Surface and cartoon models are shown on the left and right, respectively. Contacting residues are labeled on the right. (B) Split-Renilla luciferase dimerization assay of WT p65 and the indicated mutants. (C) The effects of mutations in E285 and Q287 on p65 on transcriptional activity were determined as described for Fig. 1A. The bars represent the means and SE of the results of 3 independent experiments. Expression of the WT and mutant proteins is shown at the bottom. (D) Dose response to WFA of the WT p65 and E285A Q287A mutant N/C-RL pairs. The results represent the means ± SE of 3 independent protein preparations. (E) Purified p65 WT or E285A Q287A (3.4 μM) was incubated with increasing concentrations of WFA, and the intrinsic fluorescence levels were measured using a Monolith NT.LabelFree instrument (NanoTemper). The graph shows the changes in fluorescence intensities in response to the indicated concentrations of WFA. The MST traces are shown in Fig. S2 in the supplemental material. The asterisks denote statistically significance differences (P < 0.05).