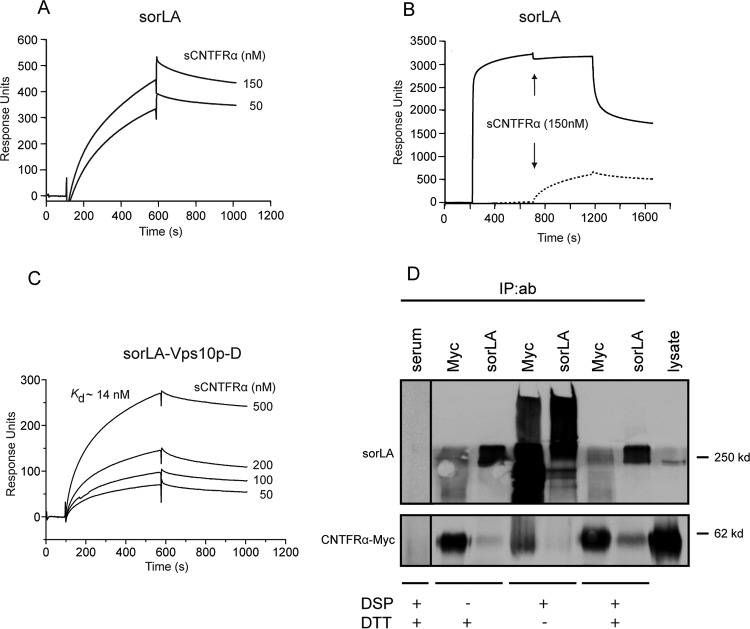
FIG 6.
Interaction between the luminal domains of CNTFRα and sorLA. (A) Concentration-dependent binding of sCNTFRα to the immobilized luminal (entire extracellular) part of sorLA. (B) RAP-mediated inhibition of the interaction between sCNTFRα and sorLA. Immobilized sorLA was exposed to unsupplemented buffer (dotted line) or to buffer supplemented with 5 μM RAP (solid line) prior to the addition of sCNTFRα (arrows). (C) Concentration dependence of sCNTFRα binding to the immobilized Vps10p-D of sorLA. (D) Cross-linking of full-length receptors in cells. HEK293 double transfectants expressing CNTFRα-Myc and sorLA were incubated with the chemical cross-linker DSP (45 min, 2 nM), and receptor proteins were precipitated from lysates using anti-Myc and anti-sorLA antibodies as indicated. Reduced (+DTT) and unreduced samples of precipitate were analyzed by SDS-PAGE. IP, immunoprecipitation; ab, antibody.