Abstract
Platelet-derived exosomes mediate platelet atherogenic interactions with endothelial cells and monocytes. A new method for isolation of plasma platelet-derived exosomes is described and used to examine effects of aging and aspirin on exosome cargo proteins. Exosome secretion by purified platelets in vitro did not increase after exposure to thrombin or collagen, as assessed by exosome counts and quantification of the CD81 exosome marker. Thrombin and collagen increased exosome content of α-granule chemokines CXCL4 and CXCL7 and cytoplasmic high-mobility group box 1 (HMGB1) protein, but not membrane platelet glycoprotein VI (GPVI), with dependence on extracellular calcium. Aspirin consumption significantly blocked thrombin- and collagen-induced increases in exosome cargo levels of chemokines and HMGB1, without altering total exosome secretion or GPVI cargo. Plasma platelet-derived exosomes, enriched by absorption with mouse antihuman CD42b [platelet glycoprotein Ib (GPIb)] mAb, had sizes and cargo protein contents similar to those of exosomes from purified platelets. The plasma platelet-derived exosome number is lower and its chemokine and HMGB1 levels higher after age 65 yr. Aspirin consumption significantly suppressed cargo protein levels of plasma platelet-derived exosomes without altering total levels of exosomes. Cargo proteins of human plasma platelet-derived exosomes may biomark platelet abnormalities and in vivo effects of drugs.— Goetzl, E. J., Goetzl, L., Karliner, J. S., Tang, N., Pulliam, L. Human plasma platelet-derived exosomes: effects of aspirin.
Keywords: chemokines, glycoproteins, HMGB1, intercellular communication, atherosclerosis
Human platelets generate 50- to 120- nm-diameter exosomes that are secreted from endosomally derived multivesicular bodies, with lesser contributions directly from α-granules (1, 2). The constitutive release of exosomes and quantities of their protein cargoes loaded at multiple sites increase only modestly after stimulation with thrombin, collagen, or calcium ionophore . In contrast, platelet release of plasma membrane-derived, cytosol-containing 150- to 1000- nm-diameter microvesicles is strikingly enhanced by many stimuli, physicochemical stresses, and apoptosis (3). Furthermore, in contrast to the predominantly phospholipid content of microvesicles, platelet exosomal cargoes may include diverse cytokines, chemokines, growth factors, coagulation factors, lipoproteins, and other lipids, as well as several types of RNA (1–3). Platelet exosome membrane proteins also reflect those of their platelet source, including the constitutively expressed glycoprotein GPIb, as well as GPVI, αIIbβ3, CD40 ligand, and P-selectin from activated platelets (1, 3, 4).
Interactions of platelets with endothelial cells and monocytes that are important in the pathogenesis of atherosclerosis are mediated, in part, by platelet exosomes. Many studies have not distinguished between total platelet microparticles and the exosome and microvesicle subsets. However, it is platelet exosomes, like exosomes from many other types of cells, that serve as the major mediators of platelet intercellular interactions (5, 6). Preliminary findings suggest that uptake of platelet-derived exosomes by endothelial cells enhances their adhesiveness by both increasing endothelial expression of adherence proteins and decreasing endothelial generation of antiadhesive factors (4, 7, 8). Platelet exosomes also augment platelet adherence to monocytes and monocyte activation to an inflammatory phenotype (9).
Our hypothesis is that sustained platelet activation in vivo in some vascular diseases will elevate loading of cytoadhesive, thrombogenic, and inflammatory factors into platelet exosomal cargo sufficiently to promote their greater delivery to endothelial cells and macrophages at sites of vascular lesions. Augmented delivery of platelet exosomal atherogenic cargo to lesional endothelial cells and macrophages may consequently accelerate development of vascular plaques, clots, and strictures (10, 11).
MATERIALS AND METHODS
Characteristics of subjects
Each subject was healthy, lacked any history of coronary or cerebral vascular disease, had taken no platelet-active medication for at least 4 wk, and signed an informed consent, according to the protocol approved by the University of California, San Francisco Committee for Human Research. Subject ages ranged from 25 to 75 yr; 12 were men, and 12 were women.
Preparation of platelets and platelet-poor plasma
Venous blood (12 ml) was drawn into 20 ml plastic syringes from individuals who had not ingested aspirin or other platelet-active drugs in the preceding month. Portions of blood (6 ml) were added to each of 2 tubes containing acid citrate dextrose solution (Thermo Fisher Scientific, Hanover Park, IL, USA). After centrifugation at 200 g for 20 min at 20°C, 6 ml total of platelet-rich plasma from both tubes was transferred to a 15 ml plastic test tube containing 6 ml calcium- and magnesium-free Dulbecco’s balanced salt (DBS) solution with 2 mM EDTA and prostaglandin E1 (Sigma-Aldrich, St. Louis, MO, USA) at 1 µM final concentration. After centrifugation at 2200 g for 20 min at 20°C, platelet-poor plasma (PPP) was removed and frozen in 0.5 ml aliquots at −80°C. The top of each platelet pellet was rinsed with DBS solution and the platelets resuspended at 1 × 108/ml in Tyrode’s buffer (Thermo Fisher Scientific) with 3 g/L bovine serum albumin (BSA; Thermo Fisher Scientific).
Platelet stimulation and exosome isolation
Replicate 1 ml suspensions of 108 platelets were supplemented with calcium chloride to a final concentration of 1 mM and incubated at 37°C for 30 min, without and with 30 nM human plasma-derived thrombin (Sigma-Aldrich) or 0.3 µM human collagen (type 4 collagen from human placenta; Sigma-Aldrich). After centrifugation at 4000 g for 10 min at 4°C, supernates were transferred to new tubes, mixed with ExoQuick-TC solution (System Biosciences, Mountain View, CA, USA) to precipitate exosomes, held at 4°C overnight, and centrifuged at 1500 g for 30 min at 4°C. Each exosome pellet was resuspended in 50 µl DBS with protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN, USA) and phosphatase inhibitor cocktail (Halt; Pierce, Thermo Fisher Scientific). After removal of 5 µl for exosome counting, 205 µl M-PER mammalian protein extraction reagent (Thermo Fisher Scientific), with the same inhibitor cocktails, was added, followed by freezing in 50 µl aliquots at −80°C.
Isolation of platelet-derived exosomes from PPP
Replicate 0.25 ml portions of PPP were incubated for 45 min at 20°C with 0.1 ml thromboplastin-D (Thermo Fisher Scientific), received 0.15 ml DBS with protease inhibitor and phosphatase inhibitor cocktails, and then were centrifuged at 3000 g for 15 min at room temperature (12). Total exosomes were sedimented from the 3000 g supernatant with ExoQuick solution (System Biosciences) and were resuspended in 0.50 ml of 30 g/L BSA (Thermo Fisher Scientific) with protease inhibitor and phosphatase inhibitor cocktails. After reprecipitation with ExoQuick solution and recentrifugation to eliminate residual microvesicles, total exosomes were resuspended in 0.30 ml distilled water:DBS (1:1, v:v) for immunochemical enrichment (13, 14). Platelet-derived exosomes were absorbed by 2 µg/suspension of biotinylated mouse antihuman CD42b (GPIb) IgG1 antibody (clone AK2; AbD Serotec, Raleigh, NC, USA) for 90 min at 20°C and then 10 µl Streptavidin Plus UltraLink resin in 40 µl of 30 g/L BSA (Thermo Fisher Scientific) for 30 min at 20°C with gentle, continuous mixing. After centrifugation at 400 g for 5 min at 20°C and removal of the supernate, exosomes were released from the immune complexes into 100 µl of 0.05 mM acetic acid at 4°C, followed by centrifugation at 4000 g for 10 min at 4°C and transfer of the supernate into new tubes containing 10 µl of 1 mM Tris-HCl (pH = 8.0), 25 µl of 30 g/L BSA, and 365 µl M-PER solution (Thermo Fisher Scientific) for 2 freeze-thaw cycles and storage at −80°C.
Exosome counts
Exosome suspensions from purified platelets and plasma immunoprecipitates were diluted 1:100–1:200 to permit counting in the range of 108–109/ml, with an LM10 nanoparticle tracking system (NanoSight, Amesbury, United Kingdom), as described in Fiandaca et al. (12).
ELISA quantification of exosome and platelet proteins
All samples were quantified at a 1:2 (v:v) dilution with sample diluent from the respective ELISA kits, except for assays of CXCL4 that required a 1:20–1:40 (v:v) dilution. The sources of ELISA kits for human proteins were the following: GPVI, CD81, and HMGB1 (Cusabio, American Research Products, Waltham, MA, USA); CXCL7 (Abcam, Cambridge, MA, USA); and CXCL4 (platelet factor 4; RayBiotech, Norcross, GA, USA).
Statistics
The significance of differences between levels of exosome protein analytes from unstimulated and stimulated platelets was calculated by a paired Student’s t test, and those of levels of exosome protein analytes from platelets under 2 distinct sets of conditions were calculated by a 2-sample Student’s t test.
RESULTS
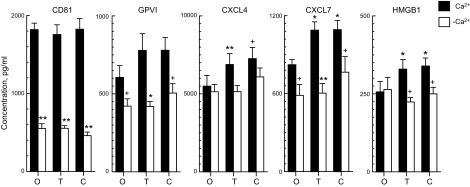
Total exosome secretion by suspensions of platelets incubated in 1 mM Ca2+-containing Tyrode’s buffer was not changed by aggregation-evoking concentrations of thrombin or collagen, as assessed by the quantities of extracted CD81 exosome marker (Fig. 1). Likewise, counts of secreted exosomes/milliliter in the same studies showed no stimulus-induced differences at 5.76 × 1010 ± 0.53 × 1010 (means ± sem, n = 6) without additive, 5.50 × 1010 ± 0.46 × 1010 with thrombin, and 5.84 × 1010 ± 0.51 × 1010 with collagen. Platelet-derived exosomes had mean and modal diameters (±sem) of 119 ± 4.0 and 87.2 ± 6.0 nm, respectively, that are similar to those of exosomes from other cells and exclude a significant presence of much larger plasma membrane microvesicles. Cargo of platelet-derived exosomes contained the platelet membrane marker GPVI, platelet α-granule markers CXCL4 and CXCL7, and the platelet cytoplasmic marker HMGB1 protein (15–18). After normalization to the same amount of exosomes by the quantity of CD81, levels of α-granule cargo proteins CXCL4 and CXCL7 and of HMGB1, but not of the membrane protein GPVI, were increased significantly by thrombin and collagen (Fig. 1). In the absence of extracellular Ca2+, total exosome secretion, reflected in the levels of CD81 and the CD81-normalized levels of cargo proteins GPVI and CXCL7 but not of CXCL4 or HMGB1, was decreased significantly, by up to 75% (Fig. 1). Furthermore, the stimulatory effects of thrombin and collagen on cargo levels of CXCL4, CXCL7, and HMGB1 were eliminated without Ca2+. Exosome counts per millilter also were decreased significantly (P < 0.001) without extracellular Ca2+ to levels of 1.17 × 1010 ± 0.05 × 1010 (means ± sem, n = 6), 1.17 × 1010 ± 0.08 × 1010, and 1.25 × 1010 ± 0.09 × 1010, respectively, without and with thrombin or collagen.
Figure 1.
Calcium dependence of platelet exosome secretion and exosome cargo levels in vitro. Each column and error bar depicts the mean ± sem for results with platelets from 6 healthy subjects. Values for GPVI, CXCL4, CXCL7 and HMGB1 were normalized for CD81 levels in the same samples. O, no additive; T, thrombin added; C, collagen added. Statistical symbols over the left, shaded columns show significance of difference between level with and without additive, determined by a paired Student’s t test. Statistical symbols over the right, open columns show significance of difference between level with and without (−)Ca2+, determined by a 2-sample Student’s t test. +P < 0.05; *P < 0.01; **P < 0.001.
Validating replicate in vitro studies of exosome secretion by platelets from another group of 6 healthy subjects confirmed a lack of effect of thrombin or collagen on total exosome release assessed by CD81 content (Fig. 2, left columns). Counts of secreted exosomes per milliliter in the same studies again showed no differences at 4.65 × 1010 ± 0.39 × 1010 (means ± sem, n = 6) without additive, 4.88 × 1010 ± 0.27 × 1010 with thrombin, and 5.03 × 1010 ± 0.42 × 1010 with collagen. When normalized to the same quantity of platelet exosomes by CD81 content, both thrombin and collagen again significantly increased the cargo levels of CXCL4, CXCL7, and HMGB1 without altering, to the same extent, that of GPVI.
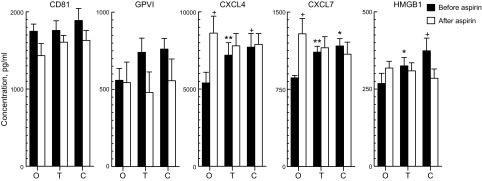
Figure 2.
Effects of aspirin consumption on platelet exosome secretion and exosome cargo levels ex vivo. Each column and error bar depicts the mean ± sem for results with platelets from 6 healthy subjects. Values for GPVI, CXCL4, CXCL7, and HMGB1 were normalized for CD81 levels in the same samples. O, no additive; T, thrombin added; C, collagen added. Statistical symbols over the shaded columns show significance of differences between levels with and without additive determined by a paired t test. Statistical symbols over the open columns show significance of differences between levels before and after aspirin consumption determined by a 2-sample t test. +P < 0.05; *P < 0.01; **P < 0.001.
Consumption of low-dose aspirin daily for 1 wk had no effect on total secretion of platelet exosomes ex vivo, as assessed by CD81 levels, nor on baseline or stimulated CD81-normalized cargo levels of GPVI (Fig. 2). Exosome counts/milliliter also were not changed significantly ex vivo by aspirin consumption at levels of 4.87 × 1010 ± 0.18 × 1010 (means ± sem, n = 6), 5.10 × 1010 ± 0.50 × 1010, and 4.84 × 1010 ± 0.10 × 1010, respectively, without and with thrombin or collagen. In contrast, this course of aspirin significantly elevated the baseline CD81-normalized cargo levels of CXCL4 and CXCL7, while concurrently blocking stimulus-induced increases in their levels and of HMGB1 (Fig. 2).
Intravascular levels of platelet exosomes were examined by a platform developed for isolation of neuronal-derived exosomes from plasma after modification for platelet specificity (12–14, 19). To test the immunoabsorptive effectiveness of a mAb to a constitutively expressed platelet surface glycoprotein, exosomes secreted by 108 platelets in vitro were precipitated with the ExoQuick-TC polymer, resuspended, and then immunoabsorbed with biotinylated mouse antihuman GPIb (CD42b) antibody plus streptavidin resin. Platelet-derived exosome recovery by immunoabsorption was a mean of 71% by levels of CD81, and after normalization for CD81 content, the relative amounts of the 4 platelet biomarkers were the same as in the initial precipitate (Table 1). Platelet exosomes from plasmas of 6 healthy subjects, which were precipitated twice by ExoQuick polymer and then isolated by this same anti-CD42b antibody immunoabsorption technique, contained levels of the CD81 exosome marker and 4 platelet cargo protein markers that were similar to those in exosomes generated by platelets in vitro (Fig. 3). Plasma platelet-derived exosomes had mean and modal diameters (±sem) of 114 ± 13.7 and 84.8 ± 11.0 nm, respectively, which were similar to those of exosomes generated in vitro. The total levels and cargo protein contents of platelet exosomes isolated from plasmas of another group of 6 healthy young subjects and 6 healthy old subjects by ExoQuick precipitation and immunoabsorption demonstrated dependence of their characteristics on aging (Table 2). Levels of total platelet exosomes in plasma were significantly lower in the older subjects, whereas cargo contents normalized for the amount of CD81 were significantly higher for CXCL4, CXCL7, and HMGB1, but not GPVI, in the older subjects.
TABLE 1.
Immunoabsorption of human platelet exosomes
| Exosome preparation | CD81 | GPVI | CXCL4 | CXCL7 | HMGB1 |
|---|---|---|---|---|---|
| Platelet exosome precipitates | 1842 ± 214 | 478 ± 24 | 9,953 ± 419 | 847 ± 22 | 422 ± 95 |
| Immunopurified platelet exosomes | 1399 ± 281* | 555 ± 26 | 10,685 ± 543 | 996 ± 73 | 438 ± 101 |
Each value is the mean ± sem pg/ml (n = 4) for proteins extracted from exosomes released by 108 platelets into 1 ml Tyrode’s buffer with 1 mM CaCl2 in 30 min at 37°C and precipitated by ExoQuick-TC (upper row) or precipitated and immunochemically purified (lower row).
The mean quantity of exosomal CD81 in immunopurified samples is significantly less than in preceding precipitates by a paired Student's t test. For the other 4 proteins, none of the differences in quantity was significant after CD81 normalization to compare for the same amount of exosomes.
P = 0.011.
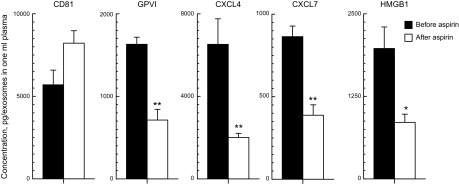
Figure 3.
Effects of aspirin consumption on plasma level of platelet-derived exosomes and their cargo levels. Each column and error bar depicts the mean ± sem for results with platelets from 6 healthy subjects. Values for GPVI, CXCL4, CXCL7, and HMGB1 were normalized for CD81 levels in the same samples. Statistical symbols over the open columns (after aspirin) show significance of differences between levels before and after aspirin consumption determined by a 2-sample t test. *P < 0.01; **P < 0.001.
TABLE 2.
Age dependence of plasma platelet exosome cargo levels
| Age range (yr) | CD81 | GPVI | CXCL4 | CXCL7 | HMGB1 |
|---|---|---|---|---|---|
| 25–35 | 6441 ± 340 | 1616 ± 64 | 5180 ± 188 | 554 ± 35 | 1104 ± 152 |
| 65–75 | 4345 ± 310 | 1601 ± 63 | 7132 ± 131 | 837 ± 66 | 2339 ± 333 |
| P | 0.0010 | 0.87 | <0.0001 | 0.0035 | 0.0071 |
Each value is the mean ± sem (pg/ml; n = 6) for platelet-derived exosomes isolated from plasma. Values for GPVI, GXCL4, CXCL7, and HMGB1 were normalized with corresponding levels of CD81. The P values (bottom row) reflect significance of the difference between the 2 age groups.
The total level of plasma platelet exosomes, assessed by the amount of CD81, was unchanged after a 1-wk course of low-dose aspirin, as was found for exosome secretion in vitro (Fig. 3). Exosome counts per milliliter of plasma similarly were not significantly altered by aspirin ingestion, at 4.32 × 1011 ± 0.55 × 1011 (means ± sem, n = 6) before and 3.32 × 1011 ± 0.79 × 1011 after. In contrast, aspirin significantly suppressed the CD81-normalized cargo levels of all platelet membrane-, granule-, and cytoplasm-derived exosome proteins in vivo (P < 0.001 for GPVI, CXCL4, and CXCL7, and P < 0.01 for HMGB1; Fig. 3). This contrasts with the effects of aspirin in vitro that did not include significant alteration of the level of membrane-derived marker GPVI (Fig. 2).
DISCUSSION
Our findings confirm that platelet exosomes have the same size, characteristic protein markers, and ratio of amount of CD81 marker-to-exosome count as exosomes from other cells (1). The largely constitutive secretion of platelet exosomes also resembles that by other types of cells, unlike the release of much larger microvesicles from platelet plasma membranes, which is greatly enhanced by platelet stimulation (Figs. 1 and 2). The current level of interest in platelet exosomes as intercellular messengers has been motivated by their high content of intrinsic proteins, RNAs, and lipid mediators that are readily delivered to other cells (5).
The many factors expected to regulate human platelet-derived exosome cargo loading, secretion, and uptake by other vascular cells are largely unexplored. Present results suggest differential specificity in the biochemical prerequisites and susceptibility to stimulation of the levels of exosome cargo proteins (Figs. 1 and 2). Total platelet exosome secretion as well as per-exosome levels of the GPVI membrane constituent, α-granule component CXCL7, and cytoplasmic protein HMGB1 but not CXCL4 depend on extracellular calcium (Fig. 1). However, exosome levels of CXCL4, CXCL7, and HMGB1 but not of GPVI or total exosome secretion are stimulated by thrombin and collagen. The effects of aspirin consumption on platelet exosomes ex vivo also include modulation of levels of CXCL4, CXCL7, and HMGB1 but not GPVI (Fig. 2). These apparently disparate aspects of platelet exosome behavior at a minimum suggest that neither protein structure nor intraplatelet localization alone is a principal determinant of regulation of protein cargo levels. Aspirin consumption reduced the in vivo plasma levels of all platelet exosomal proteins examined here, which differs from the ex vivo effects of aspirin (Figs. 2 and 3). These differences in responses to aspirin of platelet exosome cargo proteins ex vivo and in vivo indicate the likely involvement of distinctive intravascular mechanisms that may encompass physical aspects of blood flow, adhesion, and other stimuli.
Platelet HMGB1 has a distinctive role in platelet thrombotic functions. After release from first-responding platelets, HMGB1 attains concentrations that activate and aggregate other platelets and thereby, initiate a cascade of platelet thrombogenesis (17, 18). The reduction in exosome level of HMGB1 by a short course of low-dose aspirin suggests that this may be one mechanism by which such aspirin therapy may prevent platelet contributions to thrombosis and atherosclerosis.
Persistent increases in exosome delivery of multiple platelet thrombogenic and/or proinflammatory cargoes in vivo may contribute to diverse vascular pathologies, either directly or by recruitment of additional platelets or monocyte and endothelial cell pathways (7, 10). Cerebral and coronary vascular diseases leading to multiple small infarcts are the most likely vascular pathologies to involve platelet exosomes. If in vivo activation of platelets and increases in exosome thrombogenic and/or proinflammatory cargoes can be demonstrated convincingly in individuals who are predisposed to such multiple small infarcts, then application of antiplatelet activation drugs that limit exosome pathogenic effects could be justified, despite an increased risk of bleeding.
Vascular dementia is the second-most common type of senile dementia and often is a comorbidity in Alzheimer’s disease and other proteinopathic senile dementias. It is conceivable that successful treatment of proteinopathic dementia in subjects with concurrent vascular dementia might leave them little improved cognitively. However, identification and adequate treatment of concurrent vascular dementia, as well as proteinopathic dementia, may result in a greater benefit than treatment of either alone.
Acknowledgments
The authors are grateful to Judith H. Goetzl (Geriatric Research Center, Jewish Home of San Francisco) for expert preparation of the graphic illustrations. These studies were supported, in part, by Grant HL129853 (to L.P.) from the U.S. National Institutes of Health, National Heart, Lung, and Blood Institute. E.J.G. has filed a provisional patent application involving the methodology for isolation and analyses of platelet exosomes from plasma, and has also developed the methodology, obtained blood, performed exosome isolations and ELISAs, analyzed data, and drafted the manuscript; L.G. participated in study design and edited the manuscript; J.S.K. participated in study design and edited the manuscript; N.T. performed exosome counts; and L.P. analyzed data and edited the manuscript.
Glossary
- BSA
bovine serum albumin
- DBS
Dulbecco’s balanced salt
- GPIb
platelet glycoprotein Ib
- GPVI
platelet glycoprotein VI
- HMGB1
high-mobility group box 1 protein
- PPP
platelet-poor plasma
REFERENCES
- 1.Heijnen H. F., Schiel A. E., Fijnheer R., Geuze H. J., Sixma J. J. (1999) Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94, 3791–3799 [PubMed] [Google Scholar]
- 2.Aatonen M. T., Ohman T., Nyman T. A., Laitinen S., Grönholm M., Siljander P. R. (2014) Isolation and characterization of platelet-derived extracellular vesicles. J. Extracell. Vesicles 3, 24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnouf T., Goubran H. A., Chou M. L., Devos D., Radosevic M. (2014) Platelet microparticles: detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Rev. 28, 155–166 [DOI] [PubMed] [Google Scholar]
- 4.Von Hundelshausen P., Schmitt M. M. (2014) Platelets and their chemokines in atherosclerosis-clinical applications. Front. Physiol. 5, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber H. J., Holvoet P. (2015) Exosomes: emerging roles in communication between blood cells and vascular tissues during atherosclerosis. Curr. Opin. Lipidol. 26, 412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawson C., Vicencio J. M., Yellon D. M., Davidson S. M. (2016) Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J. Endocrinol. 228, R57–R71 [DOI] [PubMed] [Google Scholar]
- 7.Rautou P. E., Vion A. C., Amabile N., Chironi G., Simon A., Tedgui A., Boulanger C. M. (2011) Microparticles, vascular function, and atherothrombosis. Circ. Res. 109, 593–606 [DOI] [PubMed] [Google Scholar]
- 8.Lukasik M., Rozalski M., Luzak B., Michalak M., Ambrosius W., Watala C., Kozubski W. (2013) Enhanced platelet-derived microparticle formation is associated with carotid atherosclerosis in convalescent stroke patients. Platelets 24, 63–70 [DOI] [PubMed] [Google Scholar]
- 9.Mause S. F., von Hundelshausen P., Zernecke A., Koenen R. R., Weber C. (2005) Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler. Thromb. Vasc. Biol. 25, 1512–1518 [DOI] [PubMed] [Google Scholar]
- 10.Lievens D., von Hundelshausen P. (2011) Platelets in atherosclerosis. Thromb. Haemost. 106, 827–838 [DOI] [PubMed] [Google Scholar]
- 11.Kaplan Z. S., Jackson S. P. (2011) The role of platelets in atherothrombosis. Hematology (Am. Soc. Hematol. Educ. Program) 2011, 51–61 [DOI] [PubMed] [Google Scholar]
- 12.Fiandaca M. S., Kapogiannis D., Mapstone M., Boxer A., Eitan E., Schwartz J. B., Abner E. L., Petersen R. C., Federoff H. J., Miller B. L., Goetzl E. J. (2015) Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 11, 600–607.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapogiannis D., Boxer A., Schwartz J. B., Abner E. L., Biragyn A., Masharani U., Frassetto L., Petersen R. C., Miller B. L., Goetzl E. J. (2015) Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 29, 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goetzl E. J., Boxer A., Schwartz J. B., Abner E. L., Petersen R. C., Miller B. L., Kapogiannis D. (2015) Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 85, 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan K. L., Broekman M. J., Chernoff A., Lesznik G. R., Drillings M. (1979) Platelet α-granule proteins: studies on release and subcellular localization. Blood 53, 604–618 [PubMed] [Google Scholar]
- 16.Fiuza C., Bustin M., Talwar S., Tropea M., Gerstenberger E., Shelhamer J. H., Suffredini A. F. (2003) Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 101, 2652–2660 [DOI] [PubMed] [Google Scholar]
- 17.Yang X., Wang H., Zhang M., Liu J., Lv B., Chen F. (2015) HMGB1: a novel protein that induced platelets active and aggregation via Toll-like receptor-4, NF-κB and cGMP dependent mechanisms. Diagn. Pathol. 10, 134. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Vogel S., Bodenstein R., Chen Q., Feil S., Feil R., Rheinlaender J., Schäffer T. E., Bohn E., Frick J. S., Borst O., Münzer P., Walker B., Markel J., Csanyi G., Pagano P. J., Loughran P., Jessup M. E., Watkins S. C., Bullock G. C., Sperry J. L., Zuckerbraun B. S., Billiar T. R., Lotze M. T., Gawaz M., Neal M. D. (2015) Platelet-derived HMGB1 is a critical mediator of thrombosis. J. Clin. Invest. 125, 4638–4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetzl E. J., Boxer A., Schwartz J. B., Abner E. L., Petersen R. C., Miller B. L., Carlson O. D., Mustapic M., Kapogiannis D. (2015) Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2, 769–773 [DOI] [PMC free article] [PubMed] [Google Scholar]