FIG 5.
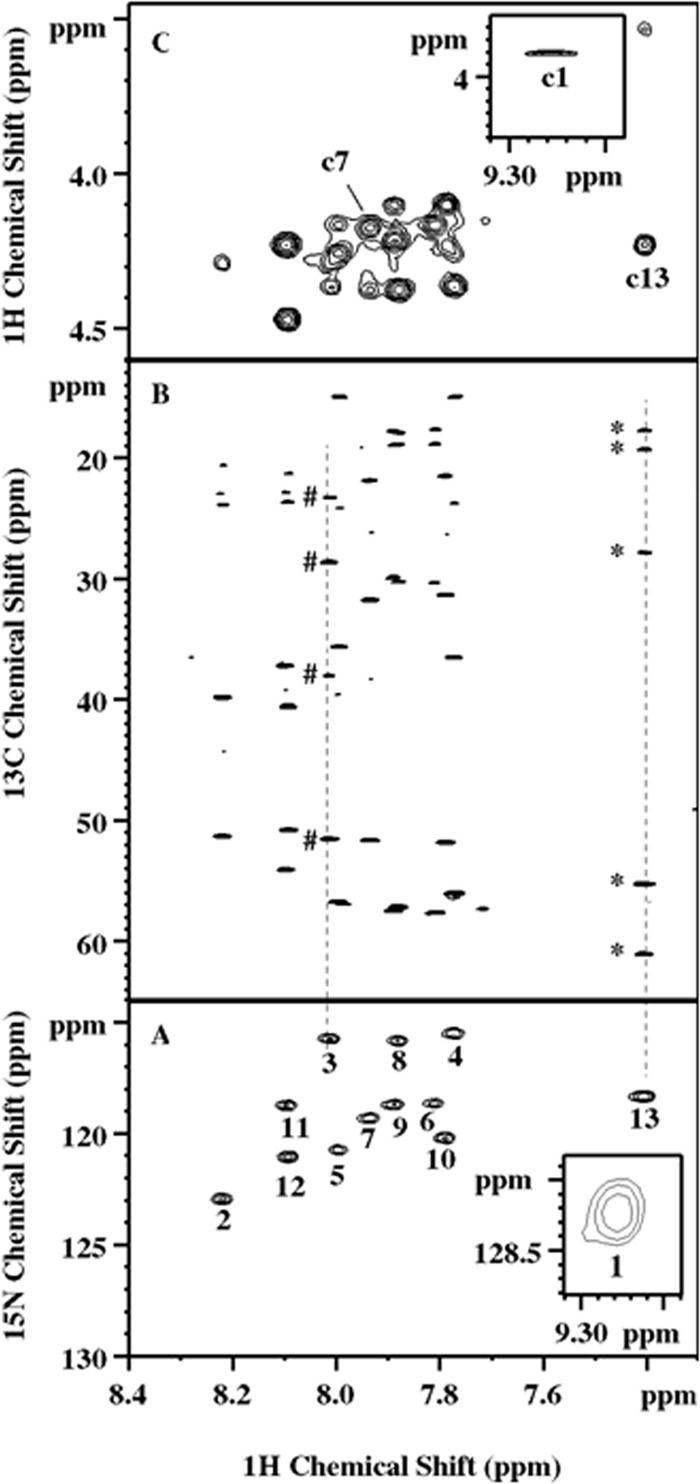
NMR spectra exemplifying the residue and sequential assignment of the lipopeptide. (A) 2D 1H-15N HSQC spectrum. (Inset) The 13 amide cross-peaks. The numbers 1 to 13 represent the order of the components in the actual final sequence. (B) 2D 1H-13C HSQC-TOCSY spectrum illustrating the identification of the side chain spin network associated with amide protons. Peaks marked by pound signs and asterisks are scalar correlated to Orn-3 HN and valinol-13 HN, respectively. Their multiplicities were determined by 2D multiplicity-edited 1H-13C HSQC as CH2, CH2, CH2, and CH for Orn-3 and CH3, CH3, CH, CH, and CH2 for valinol-13 from top to bottom, respectively, for both. (C) Partial 2D 1H-homonuclear NOESY. (Inset) Example NOE assignments of Dhb-1 HN to FA Hα (c1), Lys-7 HN to Val-6 Hα (c7), and valinol-13 HN to Leu12 Hα (c13). Ambiguities arising from a signal overlapping, for example, the region close to c7, were resolved by 2D 1H-13C HSQC-NOESY.
