Figure 4.
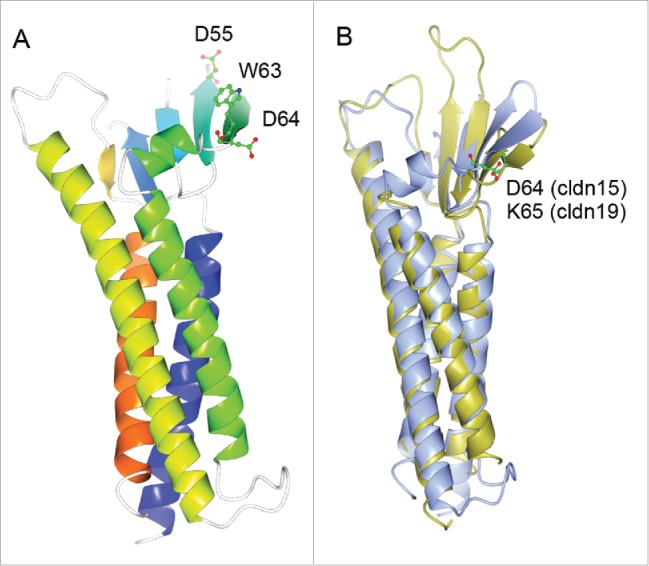
(A) 3D crystal structure of monomeric claudin-15 in ribbon representation. The color changes gradually from the N terminus (blue) to the C terminus (orange). A conserved segment of charged amino acids made of D55, W63 and D64 in the 4th β-sheet of the 1st extracellular loop are believed to form the ion permeation pore through trans-interaction. (B) Super-imposing of the crystal structure of claudin-15 onto the crystal structure of claudin-19. Claudin-15 is shown in ice blue; claudin-19 is shown in gold. The locale of the putative amino acid residue forming the permeation pore is highlighted for claudin-15 (D64) and for claudin-19 (K65) respectively.
