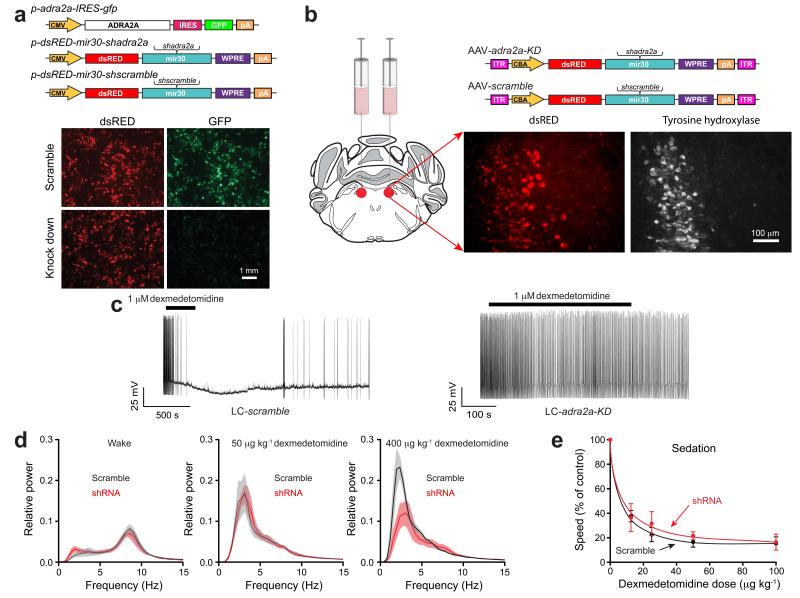
Figure 1.
Knock down of adrenergic α2A receptors in the locus coeruleus blocks dexmedetomidine-induced LORR, but not sedation. (a) Testing shRNAs for knockdown efficacy of adra2a-IRES-gfp transgene expression in HEK-293 cells (n=8). The photographs show transfected HEK-293 cells; GFP fluorescence (green) was strongly reduced with the shadra2a1 construct but not with the scramble version. The dsRED expression reveals similar transfection efficiencies. CMV, cytomegalovirus promoter/enhancer region; IRES, internal ribosome entry site; pA, polyadenylation sequence; WPRE, woodchuck post-transcriptional regulatory element. (b) AAVs expressing either dsRED-mir30-shadra2a or dsRED-mir30-shscramble transgenes were bilaterally injected into the LC of adult mice. Photographs illustrate AAV transgene expression (dsRED) in the LC as confirmed by co-staining with tyrosine hydroxylase antisera (white). ITR, inverted terminal repeats; CBA, chicken-β-actin enhancer/promoter. (c) Whole-cell recordings of action potentials of LC neurons, in acute slices from LC-scramble, and LC-adra2a-KD mice. Applying dexmedetomidine to scramble-expressing neurons hyperpolarized the membrane potential and the neurons stopped firing; by contrast dexmedetomidine had no effect on the neurons from the LC-adra2a-KD mice (P=0.7; n=7 cells). (d) Fourier transform power spectra for LC-scramble (black), and LC-adra2a-KD (red) mice in the waking state (left; n=5) and in response to 50 μg kg−1 dexmedetomidine (middle; n=4) and 400 μg kg−1 dexmedetomidine (right; n=4). Lighter shaded envelopes indicate the s.e.m. (e) Movement of LC-scramble (black; n=4-6), and LC-adra2a-KD (red; n=4–7) mice in response to sedative doses of dexmedetomidine were not significantly different (two-way ANOVA, P=0.91).