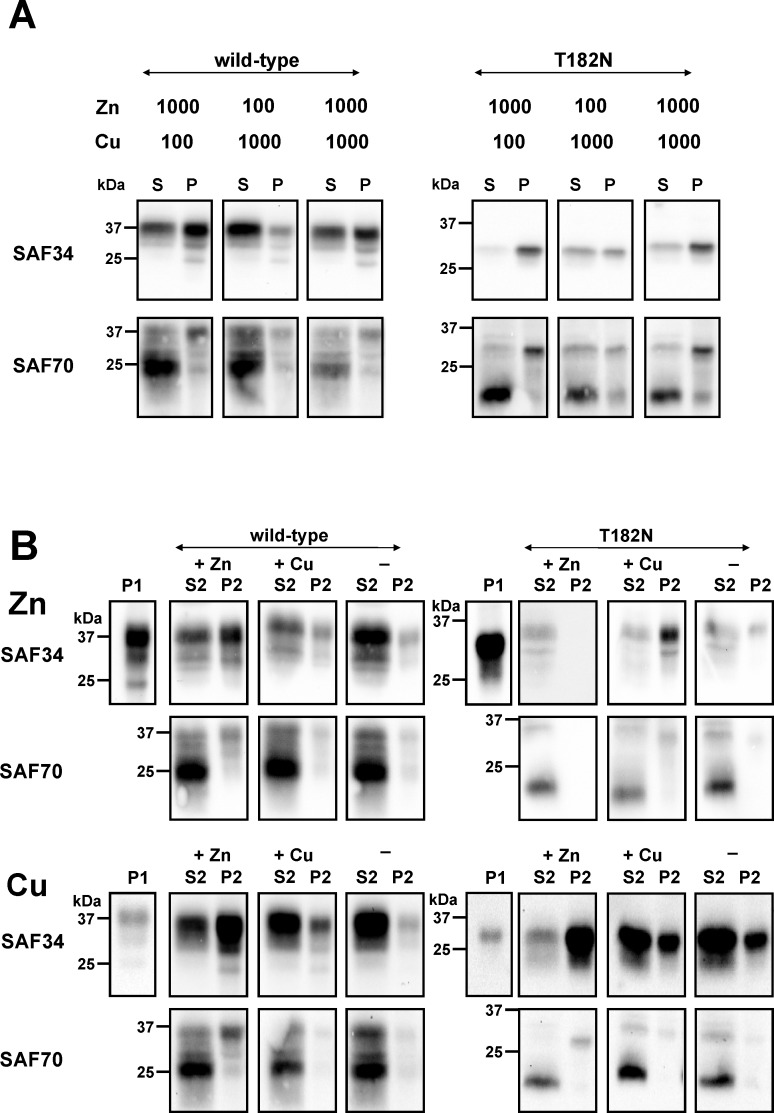
Fig 6. The PrPC–Zn2+ phenotype dominated in PrPC interactions after simultaneous and serial CuCl2 and ZnCl2 incubation.
(A) Brain homogenate proteins (10%) derived from the C57BL wild-type and T182N transgenic mice were suspended in TBS with OGP (2%) followed by simultaneous incubation with ZnCl2 (Zn) and CuCl2 (Cu) in concentrations of 100 and 1000 µM as indicated. Proteins were separated by centrifugation into a protein fraction of high solubility represented as supernatant (S) and low solubility PrPC in the pellet (P). (B)Aliquots of highly soluble proteins from C57BL wild-type and T182N transgenic mice were pre-treated with zinc (Zn) and copper (Cu) ions (1 mM) each. As a result of centrifugation, proteins were separated into a pellet fraction (P1) and into highly soluble protein fractions, which were incubated additionally with ZnCl2 (+ Zn) and CuCl2 (+ Cu; 1 mM each) or in the absence of ions (-) as controls. Highly soluble proteins were detected in the supernatant S2, whereas poorly soluble PrPC were detected in the pellet P2 after centrifugation. Copper ions were unable to alter the PrPC–Zn2+ phenotype, which would have shown a conversion of pelleted PrPC to a fraction of highly soluble proteins. Protein suspensions were separated by SDS-PAGE, immunoblotted and PrPC were detected using mabs SAF34 and SAF70 as indicated. Signals were visualized after chemiluminescence reaction on an imager.