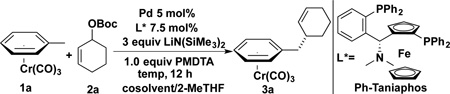
Table 1.
Optimization of the Reaction Parametersa
 | ||||||
|---|---|---|---|---|---|---|
| entry | cosolvent [%]b |
T [°C] |
Pd | conc [M] |
yield [%]c |
ee [%]d |
| 1 | THF | 0 | Pd(COD)Cl2 | 0.1 | 50 | 85 |
| 2 | 2-MeTHF | 0 | Pd(COD)Cl2 | 0.1 | 61 | 85 |
| 3 | 2-MeTHF | 25 | Pd(COD)Cl2 | 0.1 | 48 | 79 |
| 4 | 2-MeTHF | −10 | Pd(COD)Cl2 | 0.1 | 67 | 86 |
| 5 | 2-MeTHF | −20 | Pd(COD)Cl2 | 0.1 | 56 | 87 |
| 6 | Tol (30) | −10 | Pd(COD)Cl2 | 0.1 | 79 | 86 |
| 7 | Tol (30) | −10 | Pd(COD)Cl2 | 0.2 | 76 | 84 |
| 8 | Tol (30) | −10 | Pd(COD)Cl2 | 0.05 | 84 | 86 |
| 9 | Tol (30) | −10 | Pd(OAc)2 | 0.05 | 79 | 89 |
| 10 | Tol (30) | −10 | Pd(NCPh)2Cl2 | 0.05 | 64 | 86 |
| 11 | Tol (30) | −10 | Pd(dba)2 | 0.05 | 72 | 87 |
| 12 | Tol (30) | −10 | [Pd(ally)Cl]2 | 0.05 | 72 | 88 |
| 13 | Tol (30) | −20 | Pd(OAc)2 | 0.05 | 84 | 89 |
| 14 | Tol (30) | −30 | Pd(OAc)2 | 0.05 | 87 | 91 |
| 15 | Tol (30) | −40 | Pd(OAc)2 | 0.05 | 80 | 91 |
| 16 | Tol (30) | −30 | Pd(OAc)2 | 0.05 | 63 | 91e |
| 17 | Tol (30) | −30 | Pd(OAc)2 | 0.05 | 92 | 92f |
Reactions performed using 1.0 equiv. of 1a and 2 equiv. of 2a on a 0.1 mmol scale.
Cosolvent indicates 30% toluene by volume in 2-MeTHF
Yields determined by 1H NMR analysis of crude mixtures with CH2Br2 as internal standard.
The ee was determined by chiral SFC
0.5 equiv. PMDTA was used.
1.5 equiv. PMDTA was used.
