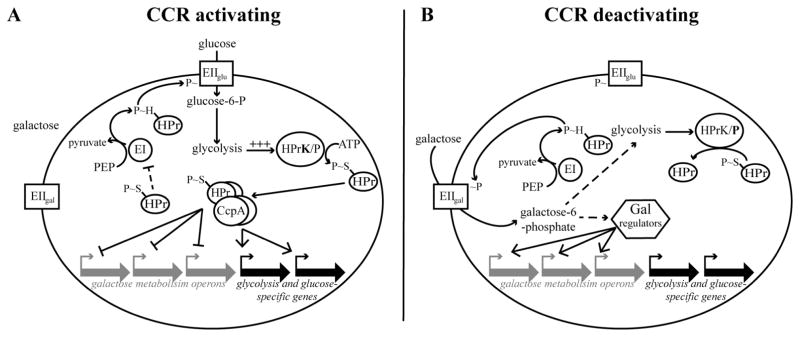
Fig. 1. Model of carbon catabolite repression in Gram-positive species.
A depicts a cell enacting carbon catabolite repression in response to a mixed carbohydrate environment; the cell is in the presence of galactose and glucose. The preferred carbohydrate glucose is efficiently transported via its phosphoenolpyruvate transport system (PTS). Glucose-6-phosphoate is then metabolized by glycolysis, causing a rapid increase in glycolytic intermediates. Glycolytic intermediates stimulate the kinase activity of HPrK/P, a dual kinase/phosphorylase. HPrK/P phosphorylates HPr at a conserved serine residue, resulting in HPr S~P. HPr S~P serves two main functions. The first function involves restricting PTS transport. HPr S~P is a poor substrate for EI-dependent phosphorylation, resulting in decreased HPr H~P in the cell. The galactose PTS is outcompeted by the glucose PTS for the remaining supply of HPr H~P. Glucose transport predominates. The second function of HPr S~P is as a co-regulator of the master CCR transcriptional regulator, CcpA. As part of this complex, CcpA recognizes pseudopalindromic sequences proximal to or within catabolite-regulated genes, resulting in repression of expression of non-preferred carbohydrate metabolism genes and activation of expression of glucose transport and metabolism genes.
B depicts the release of CCR occurring when the glucose supply is diminished. Decreased availability of glucose results in reduced glucose transport, culminating in reduced flux through glycolysis. Hence, the kinase activity of HPrK/P is no longer stimulated and it resorts to its phosphorylase activity and dephosphorylates HPr S~P. Decreased levels of HPr S~P relieve CcpA-dependent regulation. Increased availability of HPr for EI-dependent phosphorylation allows galactose transport by the galactose PTS. Galactose transport results in intracellular galactose 6-phosphate, which activates transcriptional regulators that upregulate galactose metabolism operons.