Figure 2. DNA methylation regulates synaptic scaling.
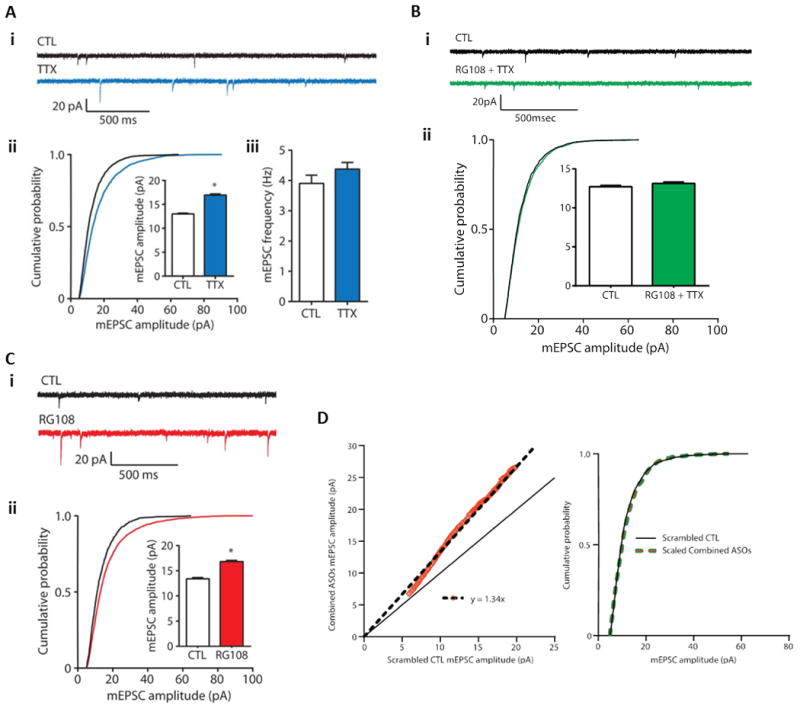
(A) Homeostatic upscaling of excitatory synaptic strength due to chronic inhibition of sodium channels with tetrodotoxin (TTX). (i) Sample spontaneous miniature excitatory post-synaptic current traces (mEPSCs) – a sample recording epoch illustrating mEPSC records from cortical pyramidal neurons after 24 hours of exposure to vehicle control (CTL) or TTX (blue) is shown. Downward spikes are postsynaptic mEPSCs triggered by glutamate release presynaptically. (ii) Cumulative probability distributions of mEPSC amplitude and overall mean mEPSC amplitudes in control and TTX-treated pyramidal neuron cultures are shown – the rightward shift of the blue line and increase in total average mEPSC amplitude are indicative of synaptic upscaling. P < 0.001, Kolmogorov-Smirnov (K-S) test. Inset: *P < 0.001, Mann-Whitney (M-W) test. (iii) Mean mEPSC frequencies do not increase in cortical pyramidal neurons treated with TTX, suggesting (as has been previously demonstrated) that the increase in (ii) is not due to an increase in total number of synaptic connections on the postsynaptic neuron. Data from cells pooled from at least six experiments for each condition (CTL, n = 21 cells; TTX, n = 15 cells). (B) Induction of excitatory synaptic scaling by TTX is blocked by DNMT inhibition. (i) Sample mEPSC traces from cortical pyramidal neurons after 24 hours of exposure to control (CTL) or TTX + the competitive DNMT inhibitor RG108 (green). (ii) Cumulative probability distributions and mean mEPSC amplitudes from cortical pyramidal neurons treated with TTX in the presence of RG108. Data are cumulative of cells pooled from at least three experiments for each condition (CTL, n = 17 cells; RG108 + TTX, n = 12 cells). These results compared to the data presented in (A) indicate that DNMT activity is necessary for TTX-induced synaptic scaling. (C) DNMT inhibition with RG108 or via DNMT mRNA knockdown upscales excitatory synaptic strength. (i) Sample mEPSC records from cortical pyramidal neurons after 24-hour treatment with control (CTL) or the DNMT inhibitor RG108 (red). (ii) Cumulative probability distributions and mean mEPSC amplitudes from cortical pyramidal neurons treated with RG108. These data indicate that DNA cytosine demethylation (known to occur secondary to DNMT inhibition) is capable of triggering synaptic upscaling. Please note that the data presented in (B) also indicate that the RG108-triggered synaptic upscaling is dependent on neuronal action potential firing, that is, that the RG108 effect is dependent on neuronal activity. Data from cells pooled from at least four experiments for each condition (CTL, n = 12 cells; RG108, n = 12 cells). P < 0.001, K-S test. Inset: *P < 0.001, M-W test. (D) Combined Dnmt1 and Dnmt3a knockdown multiplicatively upscales excitatory strength. Left Panel - Rank Order Plot of one thousand randomly selected mEPSC amplitudes from scrambled CTL and combined ASO treatment (orange values). Linear regression yielded a scaling factor of 1.34. Right Panel – Scaled-down amplitudes from combined ASO treatment were not different than scrambled CTL (K – S test, P = 0.1323). This graphical analysis illustrates that the synaptic upscaling induced by DNMT knockdown is multiplicative in nature. This finding is consistent with the hypothesis that the upscaling maintained relative individual synaptic strengths across the entire population of neuronal synapses, that is that the fractional contribution of each individual synapse was preserved and the entire neuronal synaptic population was regulated in a proportional, coordinated fashion. Bar graphs are means ± SEM. Figure and legend adapted from (Meadows et al. 2015).
