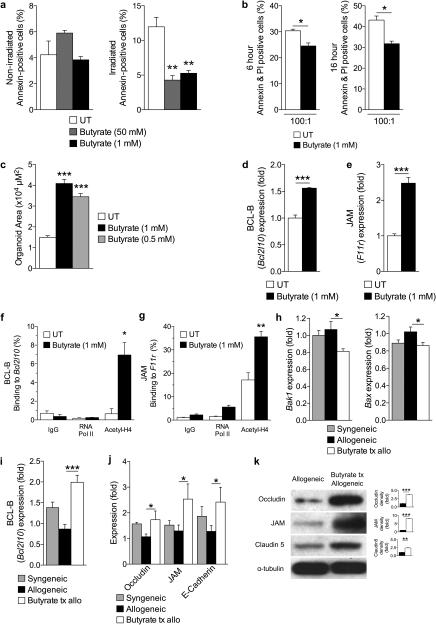
Figure 5. Butyrate treatment enhances IEC barrier.
(a) CD326+ purified intestinal epithelial cells (IECs) cultured in the presence or absence of butyrate and withheld (left panel) or subjected to (right panel) irradiation (6 Gy). (b) Allogeneic CD8+ T cell killing assay. CD326+ IECs incubated with or without butyrate overnight followed by co-culture with allo-primed CD8+ T cells. (c) Size (µm2) of cultured primary intestinal organoids following butyrate treatment (0.5 mM and 1 mM), compared to control. Gene expression of (d) anti-apoptotic protein BCL-B (Bcl2l10) and (e) junctional protein JAM (F11r) in primary CD326+ IECs in the presence of butyrate 1mM. (f)-(g) Chromatin immunoprecipitation assay (ChIP) of butyrate treated IECs (CD326+) binding acetylated histone H4 in the promoter region of (f) Bcl2l10 and (g) F11r. (h)-(i) Analysis of IECs (CD326+) isolated from recipients of syngeneic (BALB/c → BALB/c) and allogeneic (C57BL/6J → BALB/c) BMT treated with butyrate or vehicle daily via intragastric gavage for 21 days. (h) Gene expression of pro-apoptotic proteins Bak1 (left) and Bax (right), (i) anti-apoptotic protein BCL-B (Bcl2l10), and (j) junctional proteins in CD326+ purified IECs isolated 21 days following BMT. (k) Immunoblot of CD326+ purified intestinal epithelial cells from allogeneic (C57BL/6J → BALB/c) BMT recipients treated daily with intragastric vehicle or butyrate (10mg/kg) for occludin, JAM, and claudin 5; isolated 21 days following BMT. Densitometric analysis of two experiments combined shown to the right of each blot, compared to α-tubulin loading control; representative immunoblots shown. Syngeneic n=5, n = 10 mice per allogeneic group. *P < .05; **P < .01; ***P < .0001 of students t-test a – g, k; ANOVA h – j. Bars and error bars represent the means and standard errors of the mean, respectively.