Abstract
Retinal microglia (RMG) are one of the major immune cells in charge of surveillance of inflammatory responses in the eye. In the absence of an inflammatory stimulus, RMG reside predominately in the ganglion layer and inner or outer plexiform layers. However, under stress RMG become activated and migrate into the inner nuclear layer (INL) or outer nuclear layer (ONL). Activated RMG in cell culture secrete pro-inflammatory cytokines in a manner sensitive to downregulation by aldose reductase inhibitors. In this study, we utilized CX3CR1GFP mice carrying AR mutant alleles to evaluate the role of AR on RMG activation and migration in vivo. When tested on an ARWT background, IP injection of LPS induced RMG activation and migration into the INL and ONL. However, this phenomenon was largely prevented by AR inhibitors or in AR null mice, or was exacerbated in transgenic mice that over-express AR. LPS-induced increases in ocular levels of TNF-α and CX3CL-1 in WT mice were substantially lower in AR null mice or were reduced by AR inhibitor treatment. These studies demonstrate that AR expression in RMG may contribute to the proinflammatory phenotypes common to various eye diseases such as uveitis and diabetic retinopathy.
Keywords: Aldose reductase, Retinal microglia, Inflammation
1. Introduction
Microglia were discovered as a novel cell population in central nervous system (CNS) in 1933 by a Spanish physician and histologist Pío del Río-Hortega [1]. In the eye, scientists used Hortega’s staining technique to label the microglia in the retinal outer plexiform layer (OPL) in monkeys and rabbits [2], or in rats [3]. The origin of microglia has been debated for a long time. In the early 19 century, scientists hypothesized that microglia were derived from mesenchyme and used the term “mesoglia” [1], which subsequently supported by other researchers in the late 19th [4] and early 20th centuries [5]. Other observations pointed to alternative origins for microglia, including the nervous system [6, 7], monocytes/macrophages [8, 9], or even yolk sac [10–12]. Retinal microglia (RMG) migrate from the vasculature [13] and reside in the inner or outer plexiform layers, respectively. However, RMG become activated in response to proinflammatory signals or retinal injury. RMG activation has been studied in a variety of disease models, including studies of choroidal neovascularization (CNV) in wet AMD [14–16], experimental autoimmune uveoretinitis (EAU) [17, 18], and mechanisms leading to diabetic retinopathy [19]. Given the devastating vision loss associated with robust inflammation in the retina, prevention of RMG activation is an important therapeutic goal for management of many ocular diseases.
Aldose reductase (AR), best studied as an enzyme of the polyol pathway, is involved in ocular complications of diabetes including diabetic cataract [20–22] and diabetic retinopathy [23, 24]. Recent studies also implicate AR in the pathogenesis of uveitis [25–27] and fibrotic changes associated with posterior capsular opacification [28, 29]. Genetic or pharmacological blockade of AR successfully alleviates inflammatory responses induced by endotoxin [25, 26, 30–32] or hyperglycemia [33–35]. Our previous study showed that genetic or pharmacological downregulation of AR prevents endotoxin-induced inflammatory responses in RMG primary cell cultures [26]. However, understanding of AR-regulated RMG behavior in vivo is still unclear. In this study, we took a dual approach to investigate the AR effect on RMG in vivo by using AR inhibitors or AR knockout mice (ARKO). Experiments were conducted in CX3CR1GFP mice, where microglia express green fluorescent protein (GFP) [36], allowing us to easily track RMG localization and migration in the eye. By crossing the CX3CR1GFP allele with our AR Transgenic (AR-Tg) mice, we additionally tested the effect of AR expression level on RMG activation in response to endotoxin exposure. Taken together, these studies implicate AR as an effective mediator of RMG activation in the mouse eye.
2. Materials and Methods
2.1. Materials and cell culture
LPS (Salmonella enterica serotype typhimurium) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Sorbinil was generously provided by Pfizer Center Research (Groton, CT, USA. Primary RMG isolation was performed as previous described [26]. RMG were cultured in complete Dulbecco’s Modified Eagle Medium (DMEM/F12) supplemented with 4 mM L-glutamine, 10% (v/v) fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin.
2.2. Animals and treatments
This research was conducted in compliance with ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6 wildtype (WT) mice and CX3CR1GFP mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). AR transgenic mice (AR-Tg) and AR knockout (ARKO) mice were generated from previous studies [37, 38]. Animal work was approved by Institutional Animal Care and Use Committee at University of Colorado. Mice crossbred with CX3CR1GFP mice carried one copy of the CX3CR1GFP allele. All experimental mice were also genotyped as homozygous for the wild type allele of the retinal degeneration rd8 mutation [39]. For our AR inhibitor study, WT mice were randomly assigned to different treatment groups (PBS+DMSO, N = 3; LPS+DMSO, N = 3; LPS+Sorbinil (10 mg/kg in DMSO), N = 3). For AR deficiency study, WT and ARKO mice were assigned to different groups (WT+PBS, N = 3; WT+LPS, N = 3; ARKO+PBS, N = 3; ARKO+LPS, N = 3). For AR overexpression study, WT and AR-Tg mice were assigned to two groups (WT, N = 3; AR-Tg, N = 3) for studies of RMG distribution in whole retina and ocular tissue cryosections.
2.3. Immunofluorescence quantification
RMG were visualized by GFP expression in cell as previously described [36]. Retinal vasculature was visualized by staining with Isolectin IB4 Alexa Fluor conjugate (Invitrogen, Grand Island, NY, USA). In brief, enucleated eyes were embedded in Tissue-Tek optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA) for freezing. Sections (10 μm thick) were prepared by cryostat (Micro HM 550, Thermo Scientific, Waltham, MA, US) and were mounted on SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA). Sections were washed in PBS for 15 min and mounted with DAPI-mounting buffer. RMG were identified according to their GFP autofluorescence and characteristic cellular morphology. To visualize the retinal vasculature, sections were stained with Isolectin IB4 for 2 h at room temperature and then washed with PBS for 30 min. Images were obtained using a Nikon Eclipse 80i light microscope fitted to a Nikon DS Qi1Mc camera.
2.4. ELISA assay
Dissected retinas or whole eye globes were disrupted and sonicated in lysis buffer. TNF-α and CX3CL-1 amounts were determined using corresponding Mouse Cytokine and Growth Factor Immunoassays (ElisaTech, Aurora, CO). The optical density of ELISA reactions was measured using a BioTek Synergy™ 4 Hybrid Microplate Reader (Bio Tek) and the levels of each cytokine were deduced from the absorbance value by extrapolation from a standard curve generated in parallel.
2.5. Statistical analysis
Results are shown as the Means ± SEM of at least three experiments. Data were analyzed by Student’s t test with P value of <0.05 considered significant.
3. Results
3.1. Pharmacological inhibition or genetic ablation of AR alleviates LPS-induced RMG activation
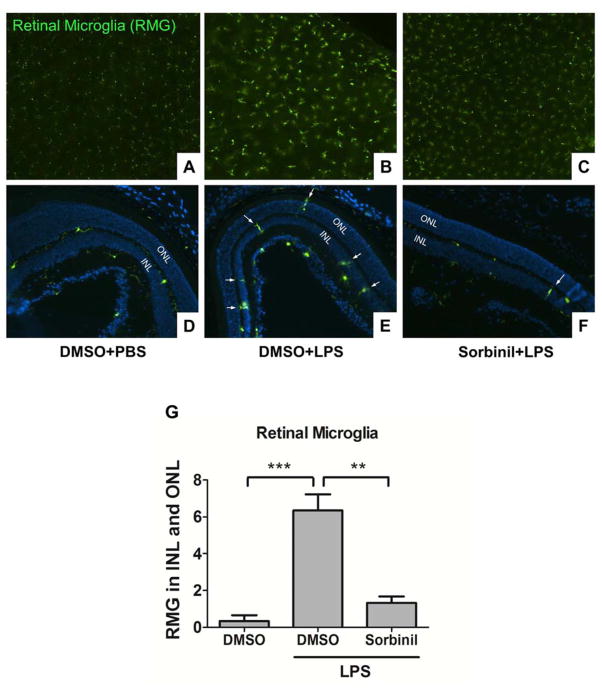
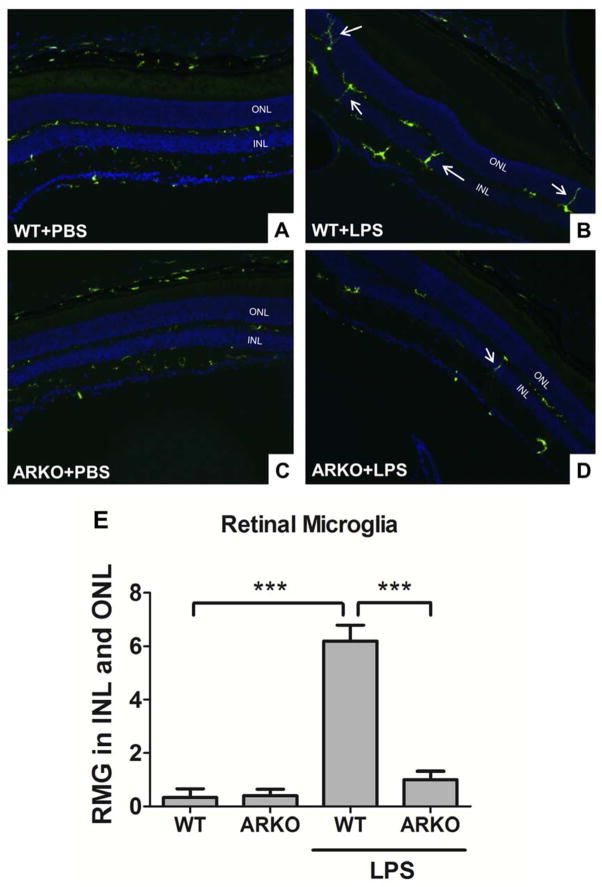
In response to inflammatory stimulation, RMG migrate from the outer plexiform layers (OPL) to the subretinal space where they release cytokines, which cause retinal damage [40]. Therefore, suppressing RMG migration is the potential strategy to protect the retina from cytokine-induced injury. Our previous study used primary cell cultures to demonstrate a role for AR in retinal inflammation. However, evaluation of the role for AR expression in RMG in the context of the complex cellular organization of the retina has not been previously reported. In this study, we utilized WT-CX3CR1GFP transgenic mice, which spontaneously express GFP in RMG in retina. At 24h following IP-injection of LPS, we observed that RMG become activated (Fig 1B) and migrate to the INL and ONL retinal layers (Fig 1E). In contrast, RMG were significantly less abundant in the INL and ONL (p<0.005) in mice treated with IP-injections of physiological saline as a control (Fig 1A and 1D). Treatment of mice with Sorbinil, a well-characterized AR inhibitor, attenuated both RMG activation (Fig 1C) and migration (Fig 1F). Cell migration in the absence or presence of AR inhibitor was combined in a bar chart (Fig 1G). As an additional test of the influence of AR on these responses to LPS stimulation, we included WT-CX3CR1GFP and ARKO-CX3CR1GFP mice for the study. In the absence of LPS, RMG in ARKO-CX3CR1GFP (Fig 2C) group behave similarly to WT-CX3CR1GFP (Fig 2A) group. In the presence of LPS, RMG migrate to INL and ONL regions of the eye (Fig 2B). However, LPS-induced RMG migration was substantially reduced in ARKO-CX3CR1GFP mice (Fig 2D) suggesting that lack of AR prevents RMG from undergoing LPS-induced activation. Significant differences in cell migration between WT and ARKO groups can be appreciated when displayed graphically as shown in Fig 2E.
Fig. 1. Aldose reductase inhibitor prevents microglia from LPS-induced activation in the retina.
WT-CX3XR1GFP mice were injected with LPS (20 mg/kg body weight) in the presence of DMSO or Sorbinil (10 mg/kg body weight) for 24 h before sacrifice. Whole retinas from each group were mounted on slides (A–C) and cryo sections (D–F) were collected from each group stained with DAPI. GFP expression was used to indicate RMG. White arrows indicate migrated RMG in inner or outer nuclear layers. Statistic data was performed in a bar chart (G). Data shown are means ± SEM (N = 3). **P < 0.01, ***P < 0.005. INL, inner nuclear layer; ONL, outer nuclear layer.
Fig. 2. Genetic ablation of aldose reductase prevents microglia from LPS-induced activation in the retina.
WT-CX3XR1GFP mice (A and B) and ARKO-CX3XR1GFP mice (C and D) were injected with LPS (20 mg/kg body weight) for 24 h before sacrifice. Cryo sections were collected from each group and stained with DAPI. GFP expression was used to indicate RMG. White arrows indicate migrated RMG in inner or outer nuclear layers. Statistic data was performed in a bar chart (F). Data shown are means ± SEM (N = 3). ***P < 0.005. INL, inner nuclear layer; ONL, outer nuclear layer.
3.2. AR regulates cytokine and chemokine production in the eye
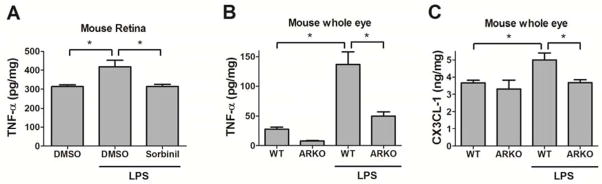
To understand the correlation of RMG activation and inflammatory modulator expression, we measured cytokine and chemokine secretion in retina and whole eye in our mouse model. We previously reported that AR modulates inflammatory product secretion in RMG [26]. In this in vivo study, we observed that genetic knockout or pharmacological inhibition of AR suppresses TNF-α production in either retina (Fig 3A) or whole eye (Fig 3B) of mice. Chemokine (C-X3-C motif) ligand 1 (CX3CL-1) is in charge of recruiting immune cells to sites of inflammation [41]. We found that LPS induces CX3CL-1 in the eye of WT mice but fails to induce CX3CL-1 elevation in the eye of ARKO mice (Fig 3C). These results are concordant with our observation of lowered LPS-induced RMG migration in the retina of ARKO mice (Fig 2).
Fig. 3. Pharmacological inhibition or genetic ablation of aldose reductase reduces LPS-induced cytokines expression in the retina and whole eye.
WT mice (A) and ARKO mice (B and C) were injected with LPS (20 mg/kg body weight) for 24 h before sacrifice. Mouse retinas (A) or whole eyes (B and C) were lysed in RIPA buffer. TNF-α (A and B) and CX3CL-1 (C) were measured using ELISA kit. Data shown are means ± SEM (N = 3). *P < 0.05.
3.3. AR overexpression elevates RMG activation in retina
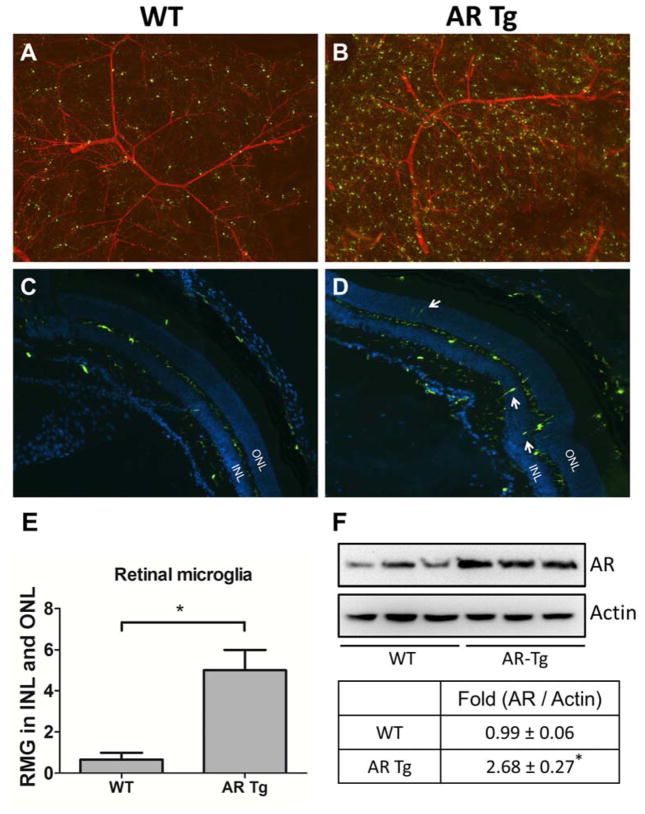
To observe the AR overexpression effect on RMG and vasculature, we utilized 3 month old ARTg-CX3CR1GFP mice in which AR is overexpressed in lens and retina. In comparison to WT mice (Fig 4A), we found an increase in number and brightness of RMG in the retinas of AR-Tg mice (Fig 4B), indicating that AR overexpression induces RMG activation. No obvious change in the retinal vasculature was noted. We observed very few RMG residing in the subretinal space (Fig 4C). However, an increased abundance of RMG was observed in inner or outer nuclear layers of retinas in AR-Tg mice (Fig 4D). Quantification of cell migration in the comparison of WT and AR-Tg groups are shown in Fig 4E. In addition, Western blots of whole retinas from WT and AR-Tg mice demonstrated that AR is elevated almost 3-fold in the AR-Tg group in comparison to WT (Fig 4F). While we do not yet know whether the elevated AR expression in retina has an endocrine rather than paracrine effect on RMG behavior, results from both genetic and pharmacological studies point to AR as playing a key role in ocular inflammation.
Fig. 4. Overexpression of aldose reductase causes microglia activation in the retina.
WT-CX3XR1GFP mice (A and C) and ARTg-CX3XR1GFP mice (B and D) were sacrificed at 3 months of age. Whole retinas from each group were mounted on slides with Isolectin IB4 staining (A and B) and cryo sections (C and D) were collected from each group stained with DAPI. GFP was spontaneously expressed indicating RMG. White arrows indicate migrated RMG in inner or outer nuclear layers. Statistic data was performed in a bar chart (E). Level of AR in retinas was measured by Western blot (F). Data shown are means ± SEM (N = 3). *P < 0.05. INL, inner nuclear layer; ONL, outer nuclear layer.
4. Discussion
Many studies have indicated that RMG are involved in ocular diseases such as uveitis [42] and diabetic retinopathy (DR) [43, 44]. Therefore, understanding the mechanism of RMG activation is a necessity for preventing these ocular diseases. Ramana and colleagues conducted many studies showing that AR plays a key role in modulating inflammatory responses in macrophages [30–32]. In agreement with Ramana’s group, we previously demonstrated that either genetic ablation or pharmacological inhibition of AR attenuates endotoxin-induced inflammatory responses in macrophages and primary RMG [25, 26]. In this study, we further demonstrated the in vivo effect of AR using AR mutant mice or studies involving AR blockade with Sorbinil, an effective AR inhibitor (Fig 1 and 2). LPS-induced RMG migration (Fig 1E and 2B) was attenuated by either Sorbinil (Fig 1F) or AR knockout (Fig 2D).
Expression levels of AR appear to correlate with the activation phenotype observed in RMG cell populations and abundance of ocular TNF-α and CX3CL-1. Levels of RMG activation and migration ability are reduced by AR inhibition (Fig 1) and gene inactivation (Fig 2). In addition, ocular TNF-α and CX3CL-1 are reduced by AR inhibition or genetic inactivation (Fig 3). The functional linkage between AR and RMG behavior is also apparent when AR levels are increased. Elevated levels of AR expression in transgenic mice are associated with increased RMG activation and migration to INL and ONL layers in the retina (Fig 4). Our observations point to AR as a drugable target for anti-inflammatory therapy in conditions such as uveitis. In addition, these studies suggest that blinding complications of diabetes, such as diabetic retinopathy and diabetic cataracts, which are associated with elevated inflammatory markers, may result from an AR-mediated mechanism [45].
AR is an enzyme involved in conversion of glucose into sorbitol using NADPH as a cofactor [46]. This AR polyol pathway generates sorbitol accumulation and NADPH depletion. NADPH participates in detoxification via glutathione (GSH) reductase pathway [47]. Therefore, reduction of NADPH attenuates removal of reactive oxygen species (ROS), suggesting that increased influx of AR polyol pathway causes oxidative stress. Diabetic patients have higher risks to develop organ complications such as nephropathy [48], cardiomyopathy [49] and peripheral neuropathy [50]. Previous data suggested that these diabetic complications could be due to AR elevation in these patients [51]. In the diabetic eye, cataract [52] and retinopathy [53] are major complications causing vision loss. Other than oxidative stress, AR polyol pathway also accelerates advanced glycation end-product (AGE) formation by producing fructose, which forms AGE much faster than glucose [54]. A study observed the increase of AGE in retina causes ocular inflammation by inducing RMG activation [19]. Accordingly, blockade of AR polyol pathway could play a protective role in retina by preventing AGE-induced inflammation.
NF-κB signaling pathway is one of the mechanisms that explain how AR induces inflammation. Many studies reported that down-regulation of AR either by genetic or enzymatic methods suppresses NF-κB activation in macrophages [30, 31] or in a mouse uveitis model [27]. Under cellular activation, NF-κB would be acetylated then translocated into nucleus to bind to transcriptional element of DNA, further inducing inflammatory signaling [55]. However, the activity of acetyl-NF-κB could be attenuated due to deacetylation by sirtulin-1, which is an NAD+-dependent deacetylase [56]. AR polyol pathway is involved in NAD+ consumption in conversion of sorbitol into fructose by sorbitol dehydrogenase (SDH) [46]. Increased influx of AR polyol pathway results in reduction of NAD+, which would be expected to reduce sirtulin deacetylase activity. We, therefore, suspect that less deacetylase activity causes more acetyl-NF-κB accumulation in nucleus and remains higher inflammatory signaling production.
Many studies showed that AR plays a role in eye pathogenesis including retinopathy. However, few studies mentioned that AR contributes to eye pathogenesis via immune cells. Here we report the first study regarding the role of AR in RMG activation in vivo. Our results point to AR as an attractive drugable target for suppressing inflammation in the eye. Future studies are required to determine if inhibition of AR is an effective strategy for prevention of ocular inflammation in a variety of disease settings such as diabetes and uveitis.
Supplementary Material
AR inhibition prevents retinal microglial activation
Endotoxin-induced ocular cytokine production is reduced in AR null mice
Overexpression of AR spontaneously induces retinal microglial activation
Acknowledgments
This study is supported by NIH grants EY005856 and EY021498.
Abbreviations
- AR
aldose reductase
- ARI
aldose reductase inhibitor
- RMG
retinal microglia
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.del Rio-Hortega P. Art and artifice in the science of histology. 1933. Histopathology. 1993;22:515–525. doi: 10.1111/j.1365-2559.1993.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 2.Vrabec F. Microglia in the monkey and rabbit retina. J Neuropathol Exp Neurol. 1970;29:217–224. [PubMed] [Google Scholar]
- 3.Ling EA. A light microscopic demonstration of amoeboid microglia and microglial cells in the retina of rats of various ages. Arch Histol Jpn. 1982;45:37–44. doi: 10.1679/aohc.45.37. [DOI] [PubMed] [Google Scholar]
- 4.Boya J, Calvo J, Prado A. The origin of microglial cells. J Anat. 1979;129:177–186. [PMC free article] [PubMed] [Google Scholar]
- 5.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 6.Hao C, Richardson A, Fedoroff S. Macrophage-like cells originate from neuroepithelium in culture: characterization and properties of the macrophage-like cells. Int J Dev Neurosci. 1991;9:1–14. doi: 10.1016/0736-5748(91)90067-v. [DOI] [PubMed] [Google Scholar]
- 7.Hutchins KD, Dickson DW, Rashbaum WK, Lyman WD. Localization of morphologically distinct microglial populations in the developing human fetal brain: implications for ontogeny. Brain Res Dev Brain Res. 1990;55:95–102. doi: 10.1016/0165-3806(90)90109-c. [DOI] [PubMed] [Google Scholar]
- 8.Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci U S A. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling EA, Penney D, Leblond CP. Use of carbon labeling to demonstrate the role of blood monocytes as precursors of the ‘ameboid cells’ present in the corpus callosum of postnatal rats. J Comp Neurol. 1980;193:631–657. doi: 10.1002/cne.901930304. [DOI] [PubMed] [Google Scholar]
- 10.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 11.Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Araya CM, Provis JM, Penfold PL, Billson FA. Development of microglial topography in human retina. J Comp Neurol. 1995;363:53–68. doi: 10.1002/cne.903630106. [DOI] [PubMed] [Google Scholar]
- 14.Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo MS, Kwak N, Ozaki H, Yamada H, Okamoto N, Yamada E, Fabbro D, Hofmann F, Wood JM, Campochiaro PA. Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am J Pathol. 1999;154:1743–1753. doi: 10.1016/S0002-9440(10)65430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010;29:500–519. doi: 10.1016/j.preteyeres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gullapalli VK, Zhang J, Pararajasegaram G, Rao NA. Hematopoietically derived retinal perivascular microglia initiate uveoretinitis in experimental autoimmune uveitis. Graefes Arch Clin Exp Ophthalmol. 2000;238:319–325. doi: 10.1007/s004170050359. [DOI] [PubMed] [Google Scholar]
- 18.Rao NA, Kimoto T, Zamir E, Giri R, Wang R, Ito S, Pararajasegaram G, Read RW, Wu GS. Pathogenic role of retinal microglia in experimental uveoretinitis. Invest Ophthalmol Vis Sci. 2003;44:22–31. doi: 10.1167/iovs.02-0199. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim AS, El-Remessy AB, Matragoon S, Zhang W, Patel Y, Khan S, Al-Gayyar MM, El-Shishtawy MM, Liou GI. Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes. 2011;60:1122–1133. doi: 10.2337/db10-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snow A, Shieh B, Chang KC, Pal A, Lenhart P, Ammar D, Ruzycki P, Palla S, Reddy GB, Petrash JM. Aldose reductase expression as a risk factor for cataract. Chem Biol Interact. 2015;234:247–253. doi: 10.1016/j.cbi.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AY, Chung SK, Chung SS. Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci U S A. 1995;92:2780–2784. doi: 10.1073/pnas.92.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anil Kumar P, Bhanuprakash Reddy G. Focus on molecules: aldose reductase. Exp Eye Res. 2007;85:739–740. doi: 10.1016/j.exer.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Obrosova IG, Kador PF. Aldose reductase / polyol inhibitors for diabetic retinopathy. Curr Pharm Biotechnol. 2011;12:373–385. doi: 10.2174/138920111794480642. [DOI] [PubMed] [Google Scholar]
- 24.Hampton BM, Schwartz SG, Brantley MA, Jr, Flynn HW., Jr Update on genetics and diabetic retinopathy. Clin Ophthalmol. 2015;9:2175–2193. doi: 10.2147/OPTH.S94508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang KC, Laffin B, Ponder J, Enzsoly A, Nemeth J, Labarbera DV, Petrash JM. Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues. Chem Biol Interact. 2013;202:283–287. doi: 10.1016/j.cbi.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang KC, Ponder J, Labarbera DV, Petrash JM. Aldose reductase inhibition prevents endotoxin-induced inflammatory responses in retinal microglia. Invest Ophthalmol Vis Sci. 2014;55:2853–2861. doi: 10.1167/iovs.13-13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav UC, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2007;48:4634–4642. doi: 10.1167/iovs.07-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang KC, Petrash JM. Aldose reductase mediates transforming growth factor beta2 (TGF-beta2)-induced migration and epithelial-to-mesenchymal transition of lens-derived epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:4198–4210. doi: 10.1167/iovs.15-16557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadav UC, Ighani-Hosseinabad F, van Kuijk FJ, Srivastava SK, Ramana KV. Prevention of posterior capsular opacification through aldose reductase inhibition. Invest Ophthalmol Vis Sci. 2009;50:752–759. doi: 10.1167/iovs.08-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramana KV, Reddy AB, Tammali R, Srivastava SK. Aldose reductase mediates endotoxin-induced production of nitric oxide and cytotoxicity in murine macrophages. Free Radic Biol Med. 2007;42:1290–1302. doi: 10.1016/j.freeradbiomed.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramana KV, Srivastava SK. Mediation of aldose reductase in lipopolysaccharide-induced inflammatory signals in mouse peritoneal macrophages. Cytokine. 2006;36:115–122. doi: 10.1016/j.cyto.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- 33.Chang KC, Snow A, LaBarbera DV, Petrash JM. Aldose reductase inhibition alleviates hyperglycemic effects on human retinal pigment epithelial cells. Chem Biol Interact. 2015;234:254–260. doi: 10.1016/j.cbi.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharya S, Manna P, Gachhui R, Sil PC. D-saccharic acid 1,4-lactone protects diabetic rat kidney by ameliorating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via NF-kappaB and PKC signaling. Toxicol Appl Pharmacol. 2013;267:16–29. doi: 10.1016/j.taap.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nuclear factor-kappaB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;53:2910–2920. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- 36.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zablocki GJ, Ruzycki PA, Overturf MA, Palla S, Reddy GB, Petrash JM. Aldose reductase-mediated induction of epithelium-to-mesenchymal transition (EMT) in lens. Chem Biol Interact. 2011;191:351–356. doi: 10.1016/j.cbi.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho HT, Chung SK, Law JW, Ko BC, Tam SC, Brooks HL, Knepper MA, Chung SS. Aldose reductase-deficient mice develop nephrogenic diabetes insipidus. Mol Cell Biol. 2000;20:5840–5846. doi: 10.1128/mcb.20.16.5840-5846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, Caspi RR. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012;53:2921–2927. doi: 10.1167/iovs.12-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS One. 2009;4:e7945. doi: 10.1371/journal.pone.0007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 42.Bousquet E, Zhao M, Ly A, Leroux Les Jardins G, Goldenberg B, Naud MC, Jonet L, Besson-Lescure B, Jaisser F, Farman N, De Kozak Y, Behar-Cohen F. The aldosterone-mineralocorticoid receptor pathway exerts anti-inflammatory effects in endotoxin-induced uveitis. PLoS One. 2012;7:e49036. doi: 10.1371/journal.pone.0049036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 44.Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971–1980. [PubMed] [Google Scholar]
- 45.Tang J, Du Y, Petrash JM, Sheibani N, Kern TS. Deletion of aldose reductase from mice inhibits diabetes-induced retinal capillary degeneration and superoxide generation. PLoS One. 2013;8:e62081. doi: 10.1371/journal.pone.0062081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrash JM. All in the family: aldose reductase and closely related aldo-keto reductases. Cell Mol Life Sci. 2004;61:737–749. doi: 10.1007/s00018-003-3402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;22:657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 48.Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nature reviews Nephrology. 2016;12:13–26. doi: 10.1038/nrneph.2015.175. [DOI] [PubMed] [Google Scholar]
- 49.Yilmaz S, Canpolat U, Aydogdu S, Abboud HE. Diabetic Cardiomyopathy; Summary of 41 Years. Korean circulation journal. 2015;45:266–272. doi: 10.4070/kcj.2015.45.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler DA. American Diabetes, Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 51.Ratliff DM, Vander Jagt DJ, Eaton RP, Vander Jagt DL. Increased levels of methylglyoxal-metabolizing enzymes in mononuclear and polymorphonuclear cells from insulin-dependent diabetic patients with diabetic complications: aldose reductase, glyoxalase I, and glyoxalase II--a clinical research center study. J Clin Endocrinol Metab. 1996;81:488–492. doi: 10.1210/jcem.81.2.8636255. [DOI] [PubMed] [Google Scholar]
- 52.Klein BE, Klein R, Wang Q, Moss SE. Older-onset diabetes and lens opacities. The Beaver Dam Eye Study. Ophthalmic Epidemiol. 1995;2:49–55. doi: 10.3109/09286589509071451. [DOI] [PubMed] [Google Scholar]
- 53.Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–2060. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- 54.Sadowska-Bartosz I, Galiniak S, Bartosz G. Kinetics of glycoxidation of bovine serum albumin by glucose, fructose and ribose and its prevention by food components. Molecules. 2014;19:18828–18849. doi: 10.3390/molecules191118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.