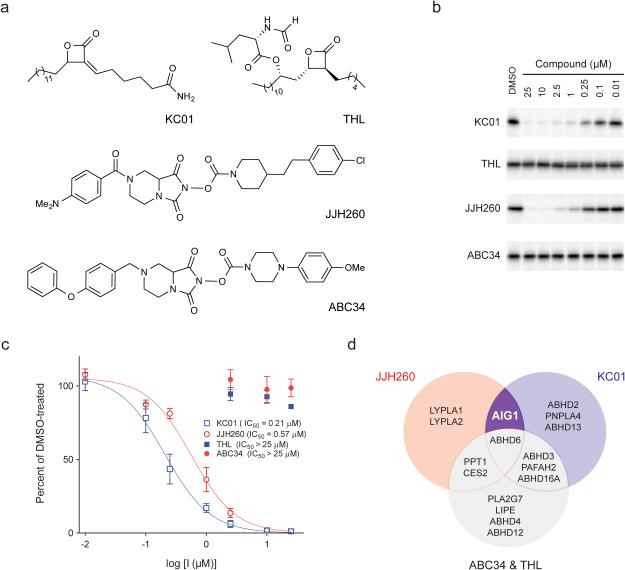
Figure 4.
Discovery of inhibitors and structurally related inactive control compounds for AIG1. (a) Chemical structures of two hAIG1 inhibitors, KC01 and JJH260, and the respective control compounds, THL and ABC34. (b) ABPP gels demonstrating inhibition of FP-Rh labeling of hAIG1 by KC01 and JJH260, but not THL and ABC34. Proteomes were treated with each inhibitor at the indicated concentrations for 30 min at 37 °C, followed by FP-Rh (1 μM, 30 min, 37 °C). Full images of the gels are provided in Supplementary Figure 18. (c) Concentration-dependent inhibition of the 9-PAHSA hydrolysis activity of hAIG1-transfected HEK293T membrane proteome by KC01 (IC50 = 0.21 ± 0.08 μM) and JJH260 (0.57 ± 0.14 μM), but not THL and ABC34. For these experiments, proteomes were incubated with each inhibitor at the indicated concentration for 30 min at 37 °C. 20 μg of each proteome was then incubated with 100 μM 9-PAHSA for 30 min at 37 °C. Data represent mean values ± s. e. m. for three biological replicates. (d) Venn diagram showing overlapping and distinct target profiles for AIG1 inhibitors KC01 and JJH260 and control compounds THL and ABC34.