Abstract
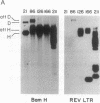
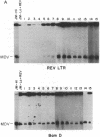
Retroviruses and herpesviruses are naturally occurring pathogens of humans and animals. Coinfection of the same host with both these viruses is common. We report here that a retrovirus can integrate directly into a herpesvirus genome. Specifically, we demonstrate insertion of a nonacute retrovirus, reticuloendotheliosis virus (REV), into a herpesvirus, Marek disease virus (MDV). Both viruses are capable of inducing T lymphomas in chickens and often coexist in the same animal. REV DNA integration into MDV occurred in a recently attenuated strain of MDV and in a short-term coinfection experiment in vitro. We also provide suggestive evidence that REV has inserted into pathogenic strains of MDV in the past. Sequences homologous to the REV long terminal repeat are found in oncogenic MDV but not in nononcogenic strains. These results raise the possibility that retroviral information may be transmitted by herpesvirus and that herpesvirus expression can be modulated by retroviral elements. In addition, retrovirus may provide a useful tool to characterize herpesviral function by insertional mutagenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht M. A., DeLuca N. A., Byrn R. A., Schaffer P. A., Hammer S. M. The herpes simplex virus immediate-early protein, ICP4, is required to potentiate replication of human immunodeficiency virus in CD4+ lymphocytes. J Virol. 1989 May;63(5):1861–1868. doi: 10.1128/jvi.63.5.1861-1868.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon L. D., Witter R. L., Fadly A. M. Augmentation of retrovirus-induced lymphoid leukosis by Marek's disease herpesviruses in White Leghorn chickens. J Virol. 1989 Feb;63(2):504–512. doi: 10.1128/jvi.63.2.504-512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G., Hayashi M., Lancz G., Tanaka A., Nonoyama M. Structure of the Marek's disease virus BamHI-H gene family: genes of putative importance for tumor induction. J Virol. 1989 Jun;63(6):2534–2542. doi: 10.1128/jvi.63.6.2534-2542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnek B. W. Marek's disease--a model for herpesvirus oncology. Crit Rev Microbiol. 1986;12(4):293–320. doi: 10.3109/10408418509104432. [DOI] [PubMed] [Google Scholar]
- Churchill A. E., Biggs P. M. Agent of Marek's disease in tissue culture. Nature. 1967 Jul 29;215(5100):528–530. doi: 10.1038/215528a0. [DOI] [PubMed] [Google Scholar]
- Churchill A. E., Payne L. N., Chubb R. C. Immunization against Marek's disease using a live attenuated virus. Nature. 1969 Feb 22;221(5182):744–747. doi: 10.1038/221744a0. [DOI] [PubMed] [Google Scholar]
- Cook M. K. Cultivation of a filterable agent associated with Marek's disease. J Natl Cancer Inst. 1969 Jul;43(1):203–212. [PubMed] [Google Scholar]
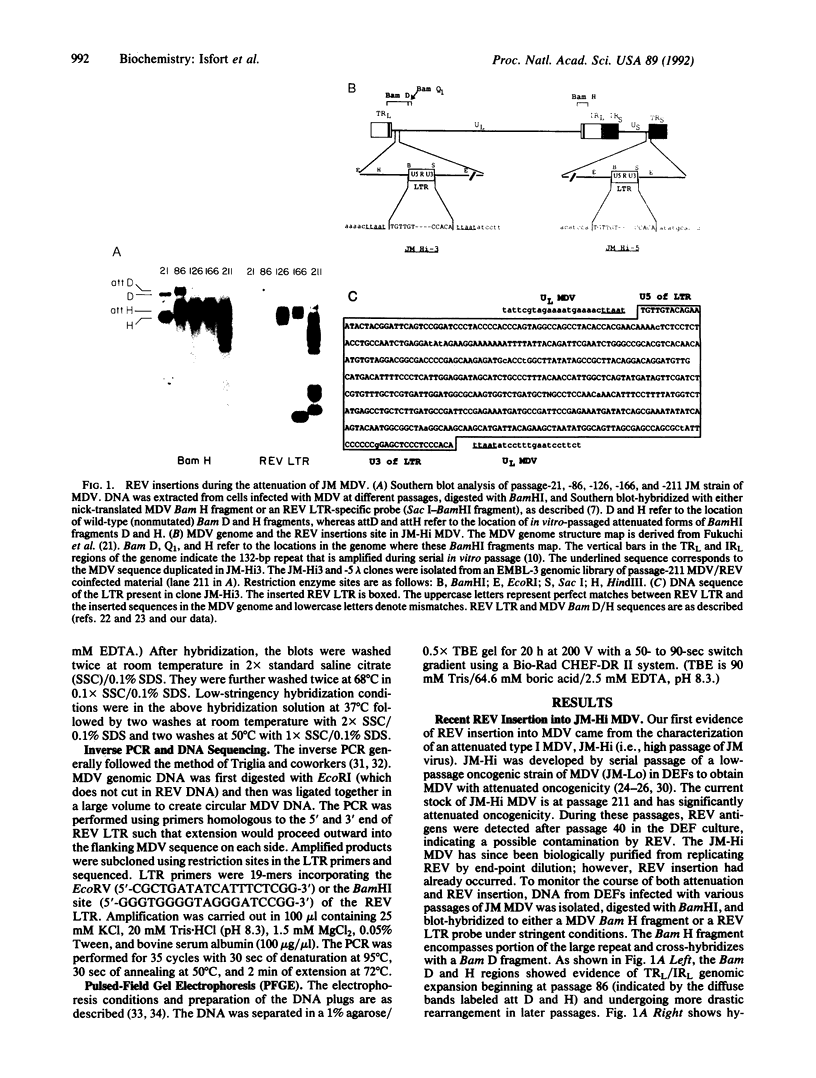
- Fukuchi K., Sudo M., Lee Y. S., Tanaka A., Nonoyama M. Structure of Marek's disease virus DNA: detailed restriction enzyme map. J Virol. 1984 Jul;51(1):102–109. doi: 10.1128/jvi.51.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Tanaka A., Schierman L. W., Witter R. L., Nonoyama M. The structure of Marek disease virus DNA: the presence of unique expansion in nonpathogenic viral DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(3):751–754. doi: 10.1073/pnas.82.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R. J., Robinson D., Kung H. J. Purification of genomic sized herpesvirus DNA using pulse-field electrophoresis. J Virol Methods. 1990 Mar;27(3):311–317. doi: 10.1016/0166-0934(90)90099-2. [DOI] [PubMed] [Google Scholar]
- Isfort R., Witter R. L., Kung H. J. C-myc activation in an unusual retrovirus-induced avian T-lymphoma resembling Marek's disease: proviral insertion 5' of exon one enhances the expression of an intron promoter. Oncogene Res. 1987;2(1):81–94. [PubMed] [Google Scholar]
- Lusso P., De Maria A., Malnati M., Lori F., DeRocco S. E., Baseler M., Gallo R. C. Induction of CD4 and susceptibility to HIV-1 infection in human CD8+ T lymphocytes by human herpesvirus 6. Nature. 1991 Feb 7;349(6309):533–535. doi: 10.1038/349533a0. [DOI] [PubMed] [Google Scholar]
- Lusso P., Ensoli B., Markham P. D., Ablashi D. V., Salahuddin S. Z., Tschachler E., Wong-Staal F., Gallo R. C. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature. 1989 Jan 26;337(6205):370–373. doi: 10.1038/337370a0. [DOI] [PubMed] [Google Scholar]
- Maotani K., Kanamori A., Ikuta K., Ueda S., Kato S., Hirai K. Amplification of a tandem direct repeat within inverted repeats of Marek's disease virus DNA during serial in vitro passage. J Virol. 1986 May;58(2):657–660. doi: 10.1128/jvi.58.2.657-660.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl K. A., Calnek B. W., Harris W. V., Schat K. A., Lee L. F. Expression of a putative tumor-associated surface antigen on normal versus Marek's disease virus-transformed lymphocytes. J Natl Cancer Inst. 1987 Nov;79(5):991–1000. [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazerian K., Solomon J. J., Witter R. L., Burmester B. R. Studies on the etiology of Marek's disease. II. Finding of a herpesvirus in cell culture. Proc Soc Exp Biol Med. 1968 Jan;127(1):177–182. doi: 10.3181/00379727-127-32650. [DOI] [PubMed] [Google Scholar]
- Notani G., Sauerbier W. Sequence instability in the long terminal repeats of avian spleen necrosis virus and reticuloendotheliosis virus. J Mol Evol. 1987;25(3):241–247. doi: 10.1007/BF02100017. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Leonard J., Weck K. E., Rabson A. B., Gendelman H. E. Activation of the human immunodeficiency virus by herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3726–3732. doi: 10.1128/jvi.61.12.3726-3732.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schat K. A., Calnek B. W. Demonstration of Marek's disease tumor-associated surface antigen in chickens infected with nononcogenic Marek's disease virus and herpesvirus of turkeys. J Natl Cancer Inst. 1978 Sep;61(3):855–857. [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Temin H. M. No apparent nucleotide sequence specificity in cellular DNA juxtaposed to retrovirus proviruses. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7357–7361. doi: 10.1073/pnas.77.12.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R. F., Witter R. L. Genomic expansion of Marek's disease virus DNA is associated with serial in vitro passage. J Virol. 1985 Jun;54(3):690–696. doi: 10.1128/jvi.54.3.690-696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Keerikatte V. Novel use of polymerase chain reaction to amplify cellular DNA adjacent to an integrated provirus. J Virol. 1989 May;63(5):1924–1928. doi: 10.1128/jvi.63.5.1924-1928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift R. A., Boerkoel C., Ridgway A., Fujita D. J., Dodgson J. B., Kung H. J. B-lymphoma induction by reticuloendotheliosis virus: characterization of a mutated chicken syncytial virus provirus involved in c-myc activation. J Virol. 1987 Jul;61(7):2084–2090. doi: 10.1128/jvi.61.7.2084-2090.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieber V. L., Zalinskis L. L., Silva R. F., Finkelstein A., Coussens P. M. Transactivation of the Rous sarcoma virus long terminal repeat promoter by Marek's disease virus. Virology. 1990 Dec;179(2):719–727. doi: 10.1016/0042-6822(90)90139-i. [DOI] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P. C., Challberg M. D., Nelson N. J., Levine M., Glorioso J. C. Inversion events in the HSV-1 genome are directly mediated by the viral DNA replication machinery and lack sequence specificity. Cell. 1988 Jul 29;54(3):369–381. doi: 10.1016/0092-8674(88)90200-0. [DOI] [PubMed] [Google Scholar]
- Witter R. L., Nazerian K., Purchase H. G., Burgoyne G. H. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus. Am J Vet Res. 1970 Mar;31(3):525–538. [PubMed] [Google Scholar]
- Witter R. L., Offenbecker L. Nonprotective and temperature-sensitive variants of Marek's disease vaccine viruses. J Natl Cancer Inst. 1979 Jan;62(1):143–151. [PubMed] [Google Scholar]
- Zander D. V., Hill R. W., Raymond R. G., Balch R. K., Mitchell R. W., Dunsing J. W. The use of blood from selected chickens as an immunizing agent for Marek's disease. Avian Dis. 1972 Apr;16(1):163–178. [PubMed] [Google Scholar]
- Zhu Z. H., Chen S. S., Huang A. S. Phenotypic mixing between human immunodeficiency virus and vesicular stomatitis virus or herpes simplex virus. J Acquir Immune Defic Syndr. 1990;3(3):215–219. [PubMed] [Google Scholar]