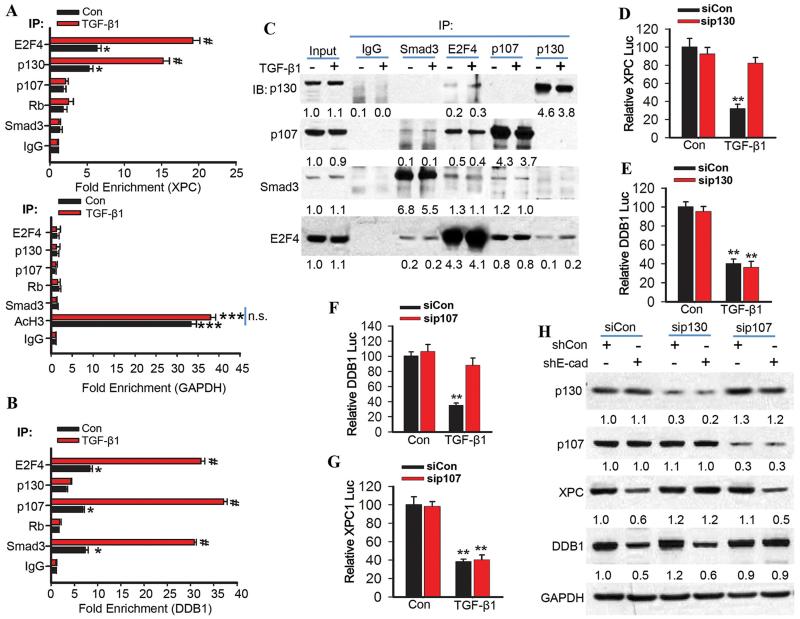
Fig. 6. The TGF-β pathway promotes binding of the E2F4/P130 complex to the XPC promoter and the E2F4/Smad3/p107 complex to the DDB1 promoter.
(A) Chromatin immunoprecipitation (ChIP) was performed in NHEK cells treated with vehicle or TGF-β1 (10 ng/ml) for 6 h using the indicated antibodies. qPCR was performed with primers specific for the XPC promoter region harboring the E2F site (upper panel) and for the human GAPDH promoter region as negative and positive control (Lower panel; AcH3, acetylated histone H3). (B) Chromatin immunoprecipitations were performed in NHEK cells treated with vehicle or TGF-β1 (10 ng/ml) for 6 h using the indicated antibodies. qPCR was performed with primers specific for the DDB1 promoter region upstream of the E2F/SP1-1/SBE-3 binding site. (C) Immunoprecipitation was performed in NHEK cells treated with vehicle or TGF-β1 (10 ng/ml) for 6 h with the indicated antibodies, followed by immunoblot analysis of p107, p130, Smad3, and E2F4. The results were obtained from three independent experiments. (D and E) Luciferase reporter assay of the XPC (−1187-27) and DDB1 promoter (−1138-124) in NHEK cells transfected with siCon or siRNA target p130 (sip130) and then treated with vehicle or TGF-β1 (10 ng/ml) for 24 h. (F and G) Luciferase reporter assay of the DDB1 (−1138-124) and XPC promoter (−1187-27) in NHEK cells transfected with siCon or siRNA target p107 (sip107) and then treated with vehicle or TGF-β1 (10 ng/ml) for 24 h. (H) Immunoblot analysis of p107, p130, XPC, DDB1, and GAPDH in HaCaT cells transfected with shCon or shE-cadherin and then transfected with siCon, sip107 or sip130. Protein levels in C and H were quantified using ImageJ software (below each band in arbitrary units). The results were obtained from three independent experiments.