Abstract
The aim of the present study was to investigate whether S-adenosylmethionine (SAM) was able to suppress activated human hepatic stellate cells (HSCs). Human LX-2 HSCs were cultured with SAM or NSC23766, and were transfected with plasmids encoding ras-related C3 botulinum toxin substrate 1 (Rac1) protein or an empty expression vector. Cell proliferation was detected by Cell Counting Kit-8. Cell migration and invasion were determined using the Transwell assay. The expression levels of Rac1 and Smad3/4 were detected by reverse transcription-quantitative polymerase chain reaction (PCR) or western blotting. The methylation status of Rac1 promoters was measured by methylation-specific PCR. The results demonstrated that SAM and NSC23766 suppressed the expression of Smad3/4 in LX-2 cells. The overexpression of Rac1 enhanced the proliferation, migration and invasion of LX-2 cells. In addition, compared with the control groups, a marked increase was observed in the protein expression levels of Smad3/4 in the LX-2 cells transfected with Rac1 plasmids. The methylation-specific PCR findings showed that SAM increased the methylation of Rac1 promoters. The results of the present study suggested that Rac1 enhanced the expression of Smad3/4 in activated HSCs; however, this increase may be suppressed by SAM-induced methylation of Rac1 promoters.
Keywords: S-adenosylmethionine, hepatic fibrosis, hepatic stellate cells, ras-related C3 botulinum toxin substrate 1, methylation
Introduction
Resident hepatic stellate cell (HSC) activation has been described as a central event in the development of hepatic fibrosis; therefore, activated HSCs are considered a major target for anti-fibrotic therapy (1). It has been reported that transforming growth factor-β 1 (TGF-β1)/Smad signaling is a pivotal pathway in hepatic fibrogenesis, and suppression of the TGF-β1/Smad signaling pathway may inhibit collagen production and eventually alleviate hepatic fibrosis (2–5). Despite recent progress in hepatic fibrosis research, the mechanism underlying hepatic fibrogenesis remains to be fully elucidated, and certain issues in the approaches to anti-fibrotic treatment still need to be addressed.
The ras-related C3 botulinum toxin substrate 1 (Rac1) signaling pathway has been reported to regulate various biological processes, including cell proliferation, apoptosis, redox signaling, and gene transcription (6–8). Previous studies have shown that the overexpression of Rac1 is a novel pathway involved in hepatic fibrogenesis (9,10). In keratinocytes, Rac1 has been reported to regulate TGF-β1-mediated epithelial cell plasticity and matrix metalloproteinase (MMP)9 production (11). In addition, Rac1 may regulate the expression of Smad2/3 in pancreatic carcinoma cells; the suppression of Rac1 weakened the transcription and phosphorylation of Smad2 but enhanced the expression of Smad3 (12). Our previous study (13) indicated that S-adenosylmethionine (SAM) reduces the expression of Rac1, Smad3 and Smad4 in activated HSCs; however, the association between Rac1 and Smads in activated HSCs has yet to be revealed.
In the present study, LX-2 cells, a cell line derived from activated human HSCs (14–18), were used to demonstrate that SAM suppresses the expression of Smad3/4 via methylating the Rac1 promoter in activated HSCs.
Materials and methods
Materials and chemicals
LX-2 cells were purchased from BioHermes Bio & Medical Technology Co., Inc. (Meishan, China). Dulbecco's modified Eagle's medium (DMEM) was purchased from Wisent Inc. (Saint-Jean-Baptiste, QC, Canada). Fetal bovine serum (FBS) was obtained from Biological Industries (Beit Haemek, Israel), and SAM was from Abbott Laboratories (Lake Bluff, IL, USA). DC Protein Assay Reagent was supplied by Bio-Rad Laboratories, Inc., (Hercules, CA, USA). Cell Counting kit-8 (CCK-8) was purchased from Dōjindo Laboratories (Kumamoto, Japan), and Matrigel Basement Membrane Matrix and Cell Culture Inserts were obtained from BD Biosciences (San Jose, CA, USA). The QuantiTech Reverse Transcription and QuantiFast SYBR Green PCR kits for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) were obtained from Qiagen (Hilden, Germany). The PrimeScript RT Master Mix for methylation-specific PCR (MSP) was purchased from Takara Bio, Inc., (Otsu, Japan). Transwell chambers were from EMD Millipore (Billerica, MA, USA). The primary monoclonal antibodies, mouse anti-human Rac1 (cat. no ab33186) and mouse anti-human Smad3/4 (cat. no. ab75512/ab130242), polyclonal rabbit anti-human β-actin (cat. no. ab8227), the polyclonal horseradish-peroxidase conjugated goat anti-mouse IgG (cat. no. ab6789) and goat anti-rabbit IgG (cat. no. ab6721) secondary antibodies were all purchased from Abcam (Cambridge, UK.)
Cell culture
LX-2 cells were maintained in DMEM (high glucose) medium supplemented with 10% FBS and 100 U/ml penicillin-streptomycin solution (Biological Industries) in an incubator containing 5% CO2. Cells were divided into three treatment groups and incubated with 0 mM, 4 mM and 6 mM of SAM for 24 h.
Cell proliferation assay
LX-2 cells were recovered from the culture flask using 0.25% trypsin and 0.1% ethylenediaminetetraacetic acid, and were then seeded onto 96-well microplates at a density of 1×104 cells/well. The cell viability was evaluated with CCK-8, according to the manufacturer's protocol, and was expressed as relative cell viability, using the following formula: Percentage of cell viability (%) = OD of treated sample/OD of control sample) × 100%. OD indicates optical density. The experiment was performed in triplicate.
Plasmid transfection
LX-2 cells were transfected with plasmids encoding Rac1 protein or an empty expression vector (Shanghai GeneChem Co., Ltd., Shanghai, China) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's protocol. The Rac1 overexpression plasmid was fluorescently-labeled. The untransfected, vector-transfected and Rac1 plasmid-transfected cells were all harvested following incubation for 48 h. Transfection efficiency was evaluated by RT-qPCR, western blotting and inverted fluorescent microscopy using an Axio Scope A1 microscope (Carl Zeiss AG, Oberkochen, Germany).
Transwell migration and invasion assays
Transwell migration and invasion assays were performed using the 8-mm pore size Transwell system. For the cell invasion assay the chambers were coated with Matrigel, according to the manufacturer's protocol. Briefly, the chambers set on 24-well cluster plates were covered with 20 µl Matrigel and were incubated for 30 min at 37°C, whereas non-coated chambers were used for the cell migration assay. LX-2 cells post-transfection and control cells were resuspended in DMEM containing 0.5% FBS, and were seeded at a density of 2×104 cells/well on the upper chamber of the Transwell. Complete medium (0.5 ml) was added to the lower chamber. Following a 24 h incubation, the inner surface of the upper chamber was gently scrubbed using a cotton bud. The cells were fixed in methanol and stained with crystal violet. Cells that traversed the Transwell membrane were counted, and images corresponding to the entire membrane surface were captured using an Olympus inverted microscope equipped with a charge-coupled device camera (CKX41-F32FL; Olympus Corp., Tokyo, Japan). Five evenly spaced fields of cells were counted in each well. The assay was performed in triplicate. A group of cells was also treated with 50 µM NSC23766 (Tocris Bioscience, Bristol, UK) for 48 h, a Rac1-specific inhibitor.
Western blot analysis
Proteins were extracted in lysis buffer [30 mM Tris, pH 7.5, 150 mM sodium chloride, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1% Nonidet P-40, 10% glycerol, and phosphatase and protease inhibitor cocktail (Roche Diagnostics, GmbH, Mannheim, Germany)]. Total proteins (30 µg) were then separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred onto polyvinylidene fluoride membranes (0.45 µl; EMD Millipore). They were quantified using a DC Protein Assay. The membranes were blocked with 5% non-fat milk and Tris-buffered saline with Tween 20 for 1 at room temperature. Next, they were probed with primary antibodies (Rac1, 1:1,000; Smad3, 1:5,000; Smad4, 1:1,000; β-actin, 1:1,000) overnight at 4°C, and were then incubated with the secondary antibodies (goat anti-mouse IgG, 1:2,000; goat anti-rabbit IgG, 1:2,000) for 1 h at room temperature. The blots were visualized using Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore). Images of the western blotting products were captured and analyzed using Quantity One V4.31 (Bio-Rad Laboratories, Inc.).
RT-qPCR
Total RNA was extracted from the cells with TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and quantified using a spectrophotometer (NanoDrop 2000; Thermo Fisher Scientific, Inc.). The samples were treated with gDNA Wipeout Buffer from the QuantiFast SYBR kit. RT was performed with 1 µg RNA in a total reaction volume of 20 µl containing 200 U Moloney murine leukemia virus reverse transcriptase, according to the manufacturer's protocol, using the following protocol: 42°C for 15 min and 95°C for 3 min. The PCR primers (β-actin, forward 5′-AGCGAGCATCCCCCAAAGTT, reverse 5′-GGGCACGAAGGCTCATCATT; Rac1, forward 5′-CCCTATCCTATCCGCAAACA, reverse 5′-CGCACCTCAGGATACCACTT; and α-smooth muscle actin (SMA), forward 5′-ACTGGGACGACGACATGGAAAAG and reverse 5′-TAGATGGGGACATTGTGGGT) were designed and validated by Sangon Biotech Co., Ltd. (Shanghai, China). qPCR was performed at 95°C for 5 min, followed by 40 cycles of amplification at 95°C for 10 sec and 60°C for 30 sec on an Applied Biosystems 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The fluorescent signals were collected during the extension phase, quantification cycle (Cq) values of the sample were calculated, and transcript levels were analyzed using the 2−ΔΔCq method (19). The results were normalized to β-actin and cDNA was prepared 30 times.
MSP
Genomic DNA (gDNA; 1 µg) isolated from LX-2 cells was extracted when the cells were treated with lysis buffer and incubated with proteinase K for 10 min, next cells were treated with bisulfite using the EZ DNA Methylation kit (Zymo Research Corp., Irvine, CA, USA), according to the manufacturer's protocol. The specific primers (methylated DNA: 164 bp PCR product, forward 5′-TTAATTAAAGTGTTGGGATGATAGAC, reverse 5′-AAATCTCTTAAACCTAAAAAACGAT; and unmethylated DNA: 164 bp PCR product, forward 5′-AATTAAAGTGTTGGGATGATAGATGT and reverse 5′-AAATCTCTTAAACCTAAAAAACAAT) were designed and synthesized by Sangon Biotech Co., Ltd. Normal lymphocytes collected from healthy human blood (Dr Xuejia Lu, Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, China) were used as the negative control, whereas normal lymphocytes treated with bisulfite-converted gDNA were used as the positive control. The modified DNA was immediately used for MSP. The PCR was carried out using PrimeScript RT Master Mix (Takara Bio, Inc.) under the following conditions: 98°C for 10 min, 53°C for 30 min, 8 cycles of 53°C for 6 min and 37°C for 30 min. The methylated and unmethylated DNA was distinguished after running on a 3% agarose gel and was stained with GelRed DNA-binding dye (Biotium, Inc., Hayward, CA, USA).
Immunofluorescence (IF)
LX-2 cells were seeded onto 24-well plates containing a sterile glass slide at a density of 2×104 cells/well. Following incubation in high-glucose DMEM medium supplemented with 10% FBS and antibiotics at 37°C in a humidified incubator containing 5% CO2 for 24 h, the cells coated on the glass slide were washed three times with cold phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 20 min at room temperature. Following blocking with 1% non-fat milk for 2 h, the cells were incubated with primary antibodies (Smad3 and Smad4) at a dilution of 1:250 overnight at 4°C. Subsequently, the slides were washed three times with 0.5% PBS-Tween 20 and incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG H&L Alexa Fluor 488 secondary antibody (cat. no. ab150117; Abcam) at a dilution of 1:1,000 at 37°C for 2 h. Eventually, the slides were washed and examined using the inverted fluorescence microscope. Five random high power field images were taken in each group.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Results are presented as the mean ± standard deviation of at least three independent experiments. The significant differences among the groups were evaluated by a Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
SAM suppresses the expression of Smad3/4 in LX-2 cells
To determine the mechanism underlying SAM-induced reversal of LX-2 HSC activation, the protein expression levels of Smad3/4 were examined in cells treated with various concentrations of SAM for 24 h. As shown in Fig. 1, the protein expression of Smad3 and Smad4 in the SAM-treated groups (4 and 6 mM SAM) was significantly decreased compared with in the control group (P<0.05), as determined by western blotting. This result indicates an inhibitory effect of SAM on the expression of Smad3/4.
Figure 1.
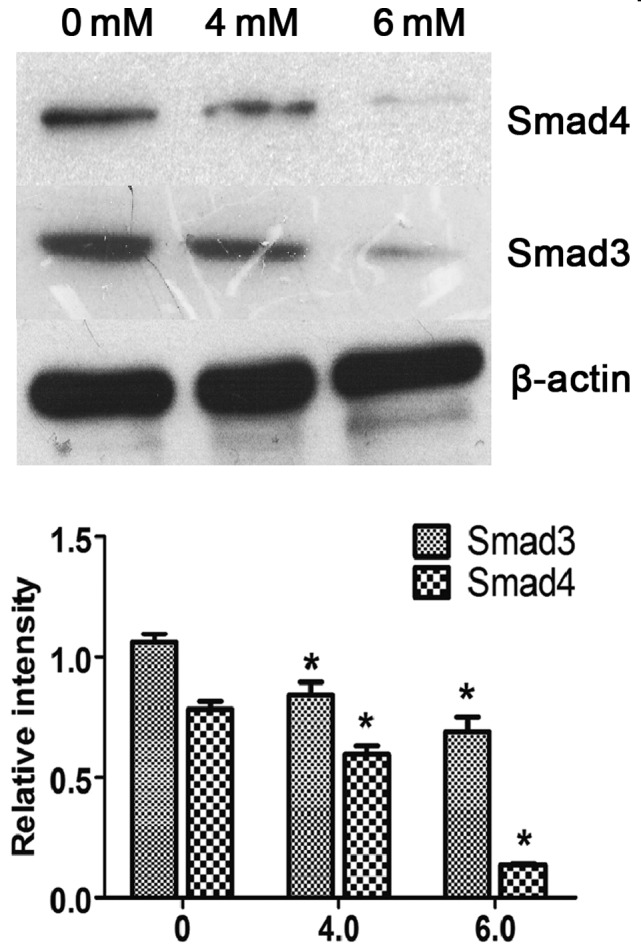
SAM suppresses the expression of Smad3/4 in LX-2 cells. Western blotting showed that the total protein of Smad3/4 in the 4 and 6 mM SAM groups was significantly decreased compared with that in the control group. Data are presented as the mean ± standard deviation. *P<0.05, compared with the control group. SAM, S-adenosylmethionine.
Rac1 enhances the proliferation, migration and invasion of LX-2 cells
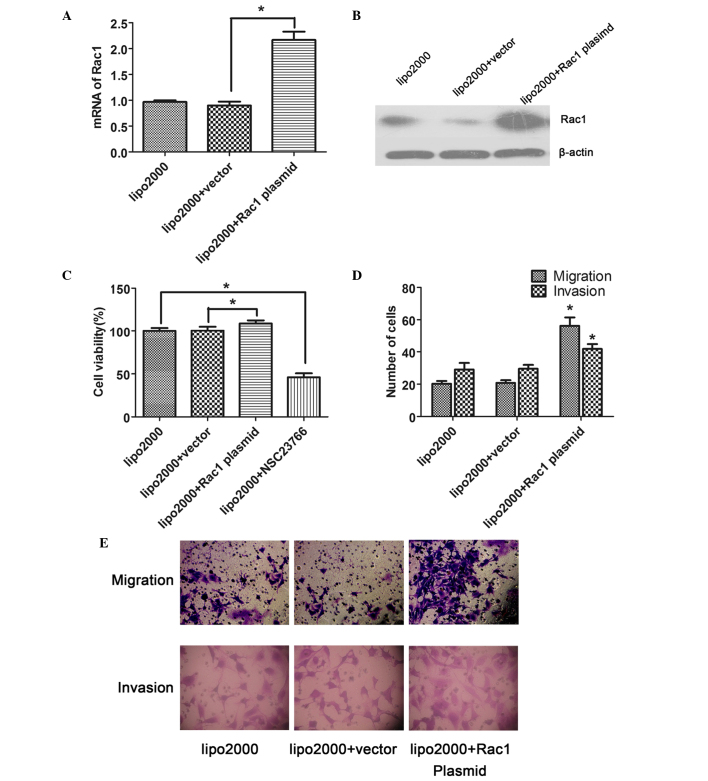
Following transfection of the cells with plasmids encoding Rac1 protein or an empty expression vector for 48 h, the expression of Rac1 in the LX-2 cells was examined. The mRNA expression levels of Rac1 in the Rac1 plasmid-transfected cells were significantly increased compared with in the vector-transfected cells, as determined by RT-qPCR (P<0.05; Fig. 2A). Similarly, the protein expression levels of Rac1 were also increased in response to Rac1 plasmid transfection (Fig. 2B), confirming that the transfection was successful.
Figure 2.
Rac1 enhances the proliferation, migration and invasion of LX-2 cells. (A and B) Reverse transcription-quantitative polymerase chain reaction and western blotting showed that the expression of Rac1 in Rac1 plasmid-transfected LX-2 cells was significantly increased compared with that in the vector-transfected cells. (C) Rac1 overexpression enhanced the proliferation of LX-2 cells, as determined by Cell Counting Kit-8, whereas the NSC23766-induced inhibition of Rac1 activity suppressed cell proliferation. (D and E) Rac1 overexpression in LX-2 cells enhanced the migration and invasion of LX-2 cells. Magnification, ×400. Data are presented as the mean ± standard deviation. *P<0.05 vs vector group. Rac1, ras-related C3 botulinum toxin substrate 1; lipo2000, Lipofectamine® 2000.
To investigate the role of Rac1 in the proliferation of LX-2 cells, CCK-8 assay was performed. The results showed that the overexpression of Rac1 significantly facilitated the proliferation of LX-2 cells compared with the vector-transfected cells (P<0.05); however, proliferation was reduced when the activity of Rac1 was suppressed by a specific inhibitor, NSC23766 (Fig. 2C).
Transwell assay was performed in order to determine the effects of Rac1 on the migration and invasion of the transfected LX-2 cells. The results indicated that the number of migrated or invaded cells was significantly increased in the Rac1 plasmid-transfected group compared with in the vector-transfected group (P<0.05; Fig. 2D and E).
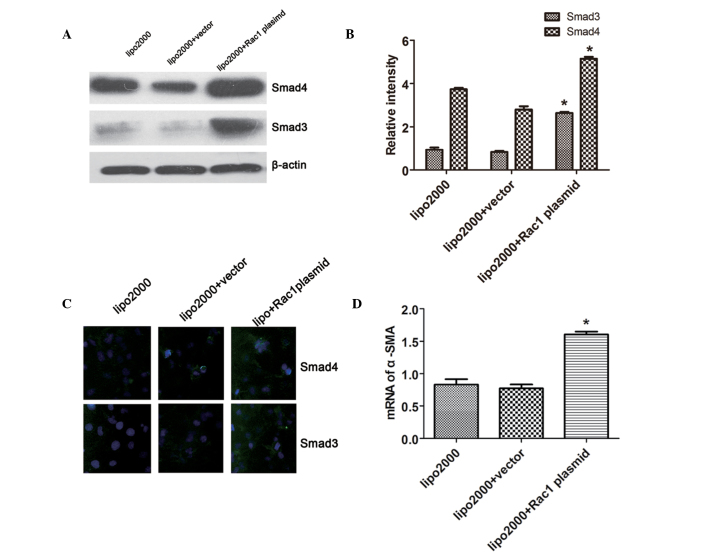
Rac1 promotes the expression of Smad3/4 in LX-2 cells
Compared with the vector-transfected cells, Rac1 plasmid-transfected LX-2 cells exhibited a marked increase in Smad3/4 protein expression (Fig. 3A and B). Smad3/4 expression detected by IF was consistent with the results of the western blot analysis (Fig. 3C). These findings indicate that Rac1 may act as part of an upstream signaling pathway that promotes the expression of Smad3/4 in LX-2 cells. In addition, the expression levels of α-SMA were detected by RT-qPCR. The mRNA expression levels of α-SMA were significantly increased in the Rac1 plasmid-transfected cells compared with in the vector-transfected cells (Fig. 3D), thus indicating that the overexpression of Rac1 facilitates the activation of HSCs.
Figure 3.
Rac1 upregulates the expression of Smad3/4 in LX-2 cells. (A and B) Western blotting indicated that the total protein of Smad3/4 in Rac1 plasmid-transfected cells was significantly increased compared with in the vector-transfected cells. (C) Smad3/4 expression was measured by immunofluorescence, and the results were consistent with those of western blotting. (D) Reverse transcription-quantitative polymerase chain reaction showed that the mRNA expression of α-SMA was upregulated by Rac1. Magnification, ×400. Data are presented as the mean ± standard deviation. *P<0.05, compared with the vector-transfected group. Rac1, ras-related C3 botulinum toxin substrate 1; α-SMA, α-smooth muscle actin; lipo2000, Lipofectamine® 2000.
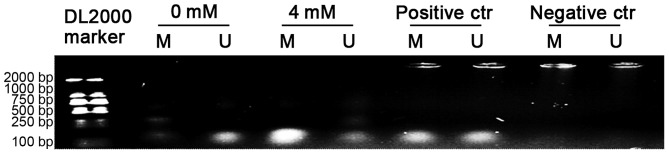
SAM increases methylation of the Rac1 promoter
gDNA was extracted from LX-2 cells treated with 4 mM SAM for 24 h, and from untreated control cells for MSP. As shown in Fig. 4, a strong signal was observed in the bisulite-converted lymphocyte DNA (positive control) amplified with both methylated and unmethylated allele-specific MSP primers, which were not effective at amplifying the negative control. A markedly increased methylation status was shown in the SAM-treated cells compared with the untreated ones, which indicated that the Rac1 promoter could be methylated by SAM in LX-2 cells, explaining the inhibitory effect of SAM on Rac1 expression.
Figure 4.
Rac1 promoter is methylated by SAM in LX-2 cells. Normal lymphocytes collected from healthy human blood were used as the negative control, whereas normal lymphocytes treated with bisulfite-converted genomic DNA were used as the positive control. Rac1 promoter was significantly methylated in the 4 mM SAM-treated group compared with in the untreated group. The polymerase chain reaction product of the Rac1 promoter was 164 bp. M, methylated; U, unmethylated; ctr, control; Rac1, ras-related C3 botulinum toxin substrate 1; SAM, S-adenosylmethionine.
Discussion
Rac1, which is a member of the Rho family of GTPases, is an important intracellular transducer. Rac1 is known to induce cytoskeletal rearrangement, and is required for cell migration, proliferation and gene transcription. Previous studies have reported that Rac1 regulates the TGF-β1/Smad signaling pathway (11,20). In addition, Rac1 may regulate the expression of Smad2/3 in pancreatic carcinoma cells, facilitating cell proliferation and mobility, whereas the suppression of Rac1 weakens the transcription and phosphorylation of Smad2 but enhances that of Smad3 (12). In keratinocytes, Rac1 has been reported to regulate TGF-β1-mediated epithelial cell plasticity and MMP9 production (11); however, the interaction between Rac1 and Smad4 has not yet been detected. Rac1 has also been shown to facilitate the synthesis of collagen, as mediated by TGF-β1 (21). Our previous study (13) demonstrated that SAM inhibited the activated phenotype of HSCs, probably via Rac1, and that SAM attenuated the expression of Smad3/4; however, the association between Rac1 and Smads in activated HSCs is currently unknown.
The present study focused on the regulating effect of Rac1 on the expression of Smad3/4 in activated HSCs, and on further demonstrating the anti-fibrotic mechanism of SAM. To validate the effects of Rac1, plasmids encoding Rac1 protein or an empty expression vector were transfected into LX-2 cells. The results demonstrated that the expression levels of Smad3 and Smad4 were markedly increased by overexpression of Rac1, which had not previously been demonstrated. Conversely, the expression levels of Smad3 and Smad4 were decreased when the expression of Rac1 was downregulated by NSC23766. Furthermore, following our previous study, the mechanism underlying the inhibitory effects of SAM on Rac1 expression was explored. SAM, which is an intermediate product of L-methionine metabolism, is a precursor of glutathione and an endogenous methyl donor. SAM regulates the expression of target genes through altering the methylation status; therefore, the present study examined the methylation status of the Rac1 promoter. The results indicated that the Rac1 promoter was highly methylated by SAM.
Smad2/3 form a hetero-oligomeric complex with Smad4, which translocates into the nucleus of HSCs where it induces target gene transcription and mediates hepatic fibrosis (1). It has previously been reported that partly suppressing the TGF-β1/Smad signaling pathway could delay or reverse hepatic fibrosis (22,23); however, a complete block of the TGF-β1/Smad pathway also increases the risk of tumorigenesis. Therefore, targeting selected Smads in TGF-β1/Smad could comprise a potential treatment for fibrosis (24).
The present study demonstrated that Rac1 may have a promising role in the regulation of Smad3/4 expression, thus revealing a novel mechanism for the reversal of liver fibrosis. The present findings suggested that the expression of Smad3/4 in activated HSCs could be enhanced by the overexpression of Rac1, whereas SAM suppressed the expression of Smad3/4, probably via methylation of the Rac1 promoter, which would result in subsequent downregulation. The present study provided a molecular basis for a potential application of SAM and Rac1 in the treatment of hepatic fibrosis.
Acknowledgments
The present study was supported by the Natural Science Foundation of Jiangsu Province (grant no. BK2011094) and the Fundamental Research Funds for the Central Universities (grant no. 20620140710).
References
- 1.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bian EB, Huang C, Wang H, Chen XX, Zhang L, Lv XW, Li J. Repression of Smad7 mediated by DNMT1 determines hepatic stellate cell activation and liver fibrosis in rats. Toxicol Lett. 2014;224:175–185. doi: 10.1016/j.toxlet.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 3.He Y, Huang C, Sun X, Long XR, Lv XW, Li J. MicroRNA-146a modulates TGF-beta1-induced hepatic stellate cell proliferation by targeting SMAD4. Cell Signal. 2012;24:1923–1930. doi: 10.1016/j.cellsig.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Wang Z, Wang J, Lam W, Kwong S, Li F, Friedman SL, Zhou S, Ren Q, Xu Z, et al. A histone deacetylase inhibitor, largazole, decreases liver fibrosis and angiogenesis by inhibiting transforming growth factor-β and vascular endothelial growth factor signalling. Liver Int. 2013;33:504–515. doi: 10.1111/liv.12034. [DOI] [PubMed] [Google Scholar]
- 5.Szuster-Ciesielska A, Mizerska-Dudka M, Daniluk J, Kandefer-Szerszeń M. Butein inhibits ethanol-induced activation of liver stellate cells through TGF-β, NFκB, p38, and JNK signaling pathways and inhibition of oxidative stress. J Gastroenterol. 2013;48:222–237. doi: 10.1007/s00535-012-0619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: A “Racˮ of all trades. Cell Mol Life Sci. 2009;66:370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagase M, Fujita T. Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol. 2013;9:86–98. doi: 10.1038/nrneph.2012.282. [DOI] [PubMed] [Google Scholar]
- 8.Elnakish MT, Hassanain HH, Janssen PM, Angelos MG, Khan M. Emerging role of oxidative stress in metabolic syndrome and cardiovascular diseases: Important role of Rac/NADPH oxidase. J Pathol. 2013;231:290–300. doi: 10.1002/path.4255. [DOI] [PubMed] [Google Scholar]
- 9.Muhanna N, Doron S, Wald O, Horani A, Eid A, Pappo O, Friedman SL, Safadi R. Activation of hepatic stellate cells after phagocytosis of lymphocytes: A novel pathway of fibrogenesis. Hepatology. 2008;48:963–977. doi: 10.1002/hep.22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bopp A, Wartlick F, Henninger C, Kaina B, Fritz G. Rac1 modulates acute and subacute genotoxin-induced hepatic stress responses, fibrosis and liver aging. Cell Death Dis. 2013;4:e558. doi: 10.1038/cddis.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santibáñez JF, Kocić J, Fabra A, Cano A, Quintanilla M. Rac1 modulates TGF-beta1-mediated epithelial cell plasticity and MMP9 production in transformed keratinocytes. FEBS Lett. 2010;584:2305–2310. doi: 10.1016/j.febslet.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Ungefroren H, Groth S, Sebens S, Lehnert H, Gieseler F, Fändrich F. Differential roles of Smad2 and Smad3 in the regulation of TGF-β1-mediated growth inhibition and cell migration in pancreatic ductal adenocarcinoma cells: Control by Rac1. Mol Cancer. 2011;10:67. doi: 10.1186/1476-4598-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Zhuge YZ, Li YJ, Gu JX. S-adenosylmethionine inhibits the activated phenotype of human hepatic stellate cells via Rac1 and matrix metalloproteinases. Int Immunopharmacol. 2014;19:193–200. doi: 10.1016/j.intimp.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K, Xu L, Eng F, Afdhal N, Kalluri R. Liver fibrosis: Insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: New tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrickson H, Chatterjee S, Cao S, Morales Ruiz M, Sessa WC, Shah V. Influence of caveolin on constitutively activated recombinant eNOS: Insights into eNOS dysfunction in BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 2003;285:G652–G660. doi: 10.1152/ajpgi.00143.2003. [DOI] [PubMed] [Google Scholar]
- 17.Werneburg NW, Yoon JH, Higuchi H, Gores GJ. Bile acids activate EGF receptor via a TGF-alpha-dependent mechanism in human cholangiocyte cell lines. Am J Physiol Gastrointest Liver Physiol. 2003;285:G31–G36. doi: 10.1152/ajpgi.00536.2002. [DOI] [PubMed] [Google Scholar]
- 18.Taimr P, Higuchi H, Kocova E, Rippe RA, Friedman S, Gores GJ. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology. 2003;37:87–95. doi: 10.1053/jhep.2003.50002. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- 21.Hubchak SC, Sparks EE, Hayashida T, Schnaper HW. Rac1 promotes TGF-beta-stimulated mesangial cell type I collagen expression through a PI3K/Akt-dependent mechanism. Am J Physiol Renal Physiol. 2009;297:F1316–F1323. doi: 10.1152/ajprenal.00345.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang LX, He RH, Yang G, Tan JJ, Zhou L, Meng XM, Huang XR, Lan HY. Asiatic acid inhibits liver fibrosis by blocking TGF-beta/Smad signaling in vivo and in vitro. PLoS One. 2012;7:e31350. doi: 10.1371/journal.pone.0031350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karaa A, Thompson KJ, McKillop IH, Clemens MG, Schrum LW. S-adenosyl-L-methionine attenuates oxidative stress and hepatic stellate cell activation in an ethanol-LPS-induced fibrotic rat model. Shock. 2008;30:197–205. doi: 10.1097/shk.0b013e318160f417. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]