Figure 2. The cSH2 domain of PLCγ mediates the recruitment of PLCγ by FGFR kinase in vitro.
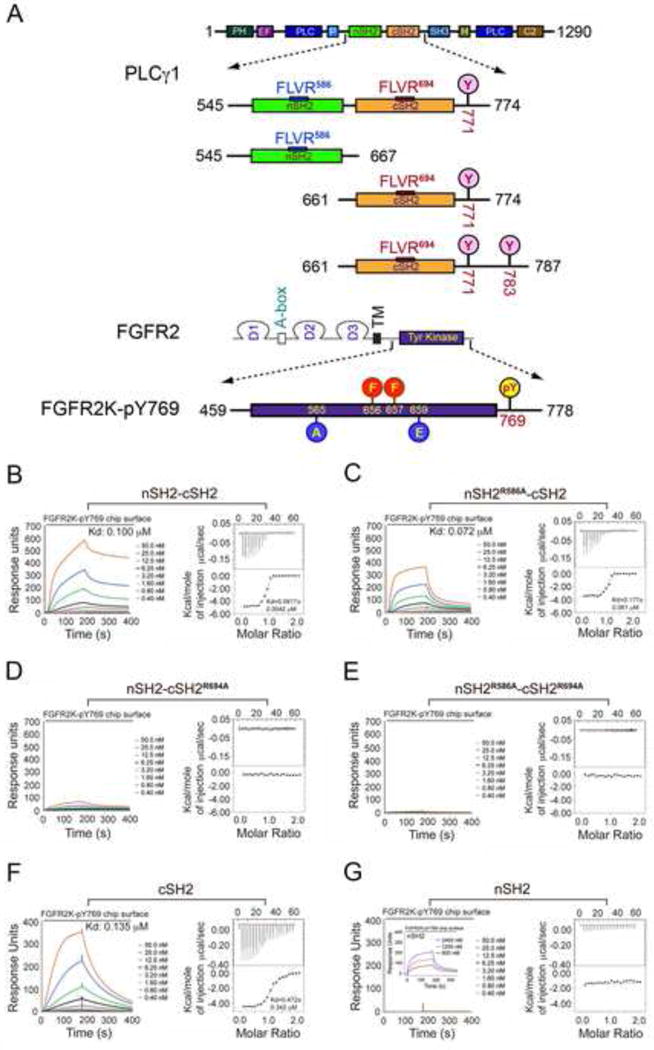
(A) Domain organization of PLCγ and FGFR2 as well as the design of the engineered PLCγ and FGFR2 fragments used in this study. Phosphorylation tyrosine sites included in the tandem SH2 and isolated cSH2 fragments are marked with pink circles. The phosphorylation sites in the A-loop of FGFR2 kinase (Tyr-656 and Tyr-657), which were mutated to phenylalanine, are indicated with red circles. The location of the two gain-of-function mutations introduced to force the kinase into the active state is indicated with blue circles. Tyr-769, the single remaining phosphorylation site in the FGFR2K is highlighted with a yellow circle. (B–G) Analysis by SPR and ITC of the recruitment of wild-type tandem nSH2-cSH2 fragment of PLCγ (B), nSH2R586A-cSH2 (C), nSH2-cSH2R694A (D), nSH2R586A-cSH2R694A (E), cSH2 domain (F) and nSH2 domain (G) of PLCγ by activated FGFR2KpY769. Insert panel of Panel G is SPR binding analysis of nSH2 domain at high concentrations with activated FGFR2KpY769. See also Figures S1 and S2.
