Figure 5. Upon recruitment via its pTyr binding pocket to the phosphorylated tail of FGFR2KpY769, the cSH2 domain experience global conformational changes that facilitate the phosphorylation of C-terminal Tyr-771 and Tyr-783 of cSH2 by another FGFR2KpY769 in trans.
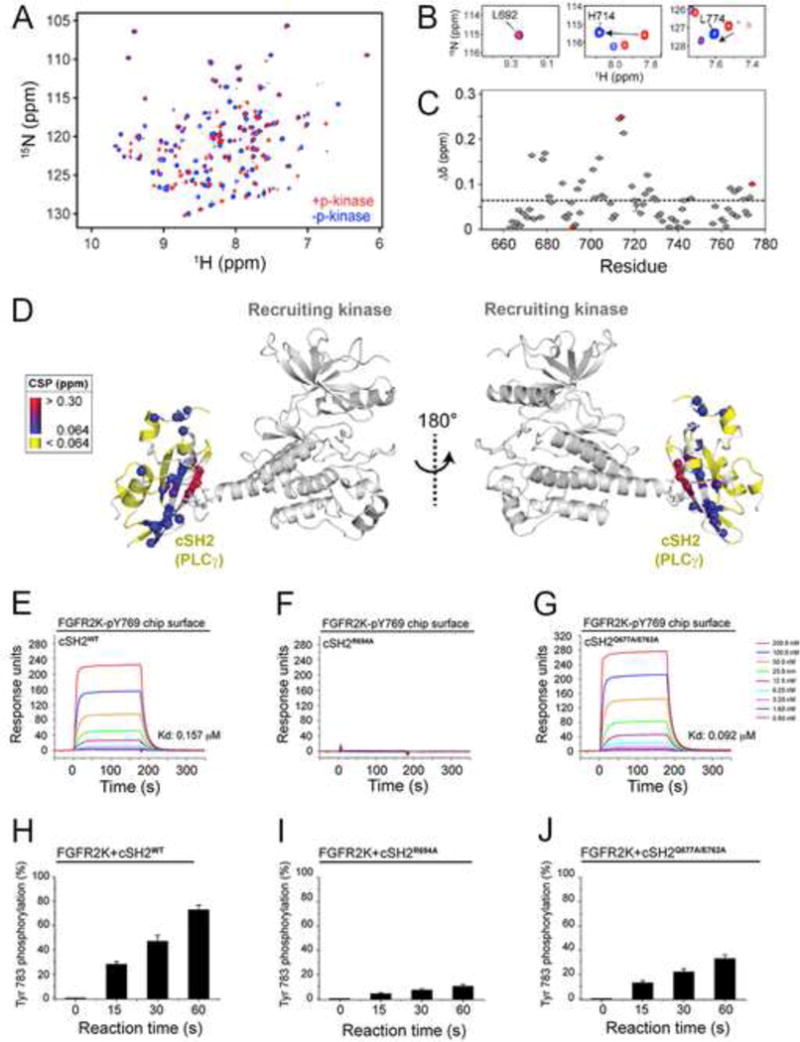
(A) Overlay of 1H/15N TROSY spectra of free cSH2 and a 1:1 complex of cSH2:FGFR2KpY769. (B) Examples of specific residues experiencing chemical shift perturbations from panel A. (C) Combined chemical shift perturbation plot showing the difference between free and bound cSH2 as a function of residue. The red circles correspond to the three residues shown in panel B. (D) Chemical shift perturbations from panel C mapped onto the structure. (E–G) Comparison of binding interactions of wild-type cSH2 domain and mutants thereof harboring R694A single and Q677A/E762A double mutations by SPR. (H–J) Comparison of phosphorylation of wild-type cSH2 domain and mutants thereof harboring R694A single and Q677A/E762A double mutations using in vitro kinas assay. In panels H–J, wild-type and mutated cSH2 domains were offered as substrate to the FGFR2KpY769 in the presence of ATP:MgCl2 and phosphorylation of cSH2 domain on Tyr-783 was quantitated using mass spectrometry. Data are represented as mean ± SEM (n=3). See also Figure S7.
