Figure 6. Mutations of PLCγ residues that interface with the “phosphorylating” kinase impair PLCγ phosphorylation without impacting PLCγ recruitment to the FGFR, PDGFR and VEGFR.
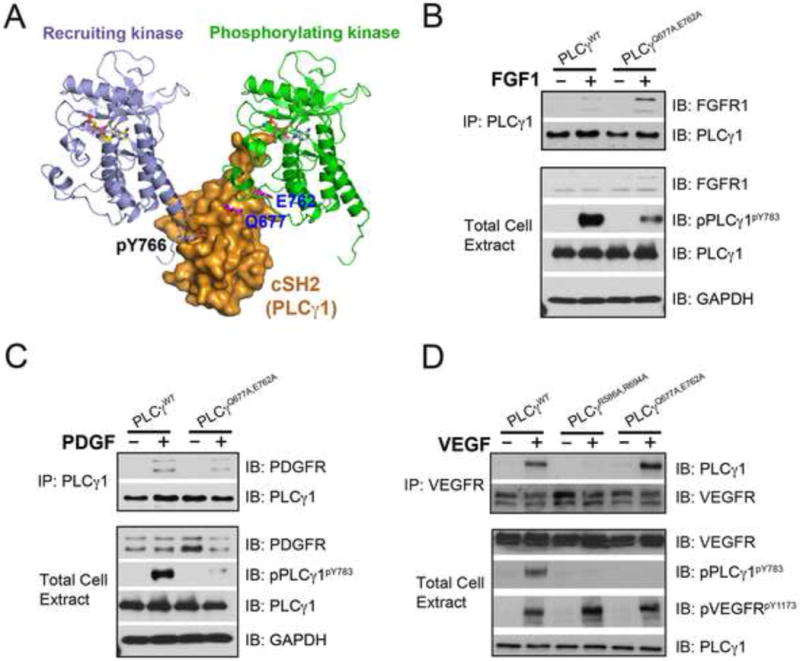
(A) Gln-677 and Glu-762 of PLCγ and the phosphotyrosine binding pocket of cSH2 domain lie on the opposing faces of cSH2 domain. (B–D) PLCγ null fibroblasts were transfected with expression vectors for wild-type PLCγ, and the indicated PLCγ mutants. Following cell stimulation with 50 ng/ml FGF1, PDGF or VEGF, cell lysates were blotted with the indicated antibodies. See also Figure S7.
