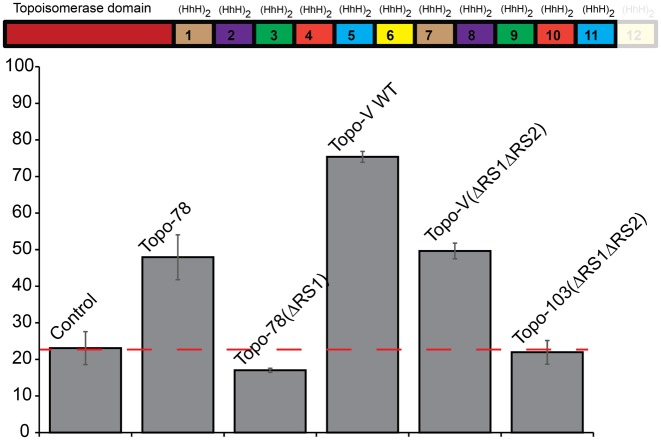
Figure 2.
Identification of a third AP lyase active site in Topo-V. (Top) Schematic representation of the Topo-V fragment used to identify the third AP lyase active site. In the fragment, Topo-103, the last (HhH)2 domain has been removed (shown shaded). (Bottom) Quantification of the AP lyase activity for full length Topo-V and the Topo-103 fragment. The wild-type protein with the first two active sites inactivated (Topo-V(ΔRS1ΔRS2)) retained partial activity, indicating the presence of a third repair site. The Topo-103(ΔRS1ΔRS2) fragment, corresponding to the Topo-103 fragment with the first two AP lyase active site mutated, showed no AP lyase activity, consistent with the existence of a third active site in the last (HhH)2 domain. The wild-type Topo-78 fragment was used as a positive control for AP lyase activity as it contains only one well-characterized repair active site. Topo-78: wild-type 78 kDa fragment; Topo-78(ΔRS1): 78 kDa fragment with three mutations to inactivate the first repair site. The partial cleavage of control DNA is due to the inherent instability of abasic DNA at the temperature used for the experiments. In all cases the level of activity reported is based on three replications and the error bars correspond to the standard error. The red dashed line corresponds to the level of cleavage of the control DNA and serves to indicate baseline activity.