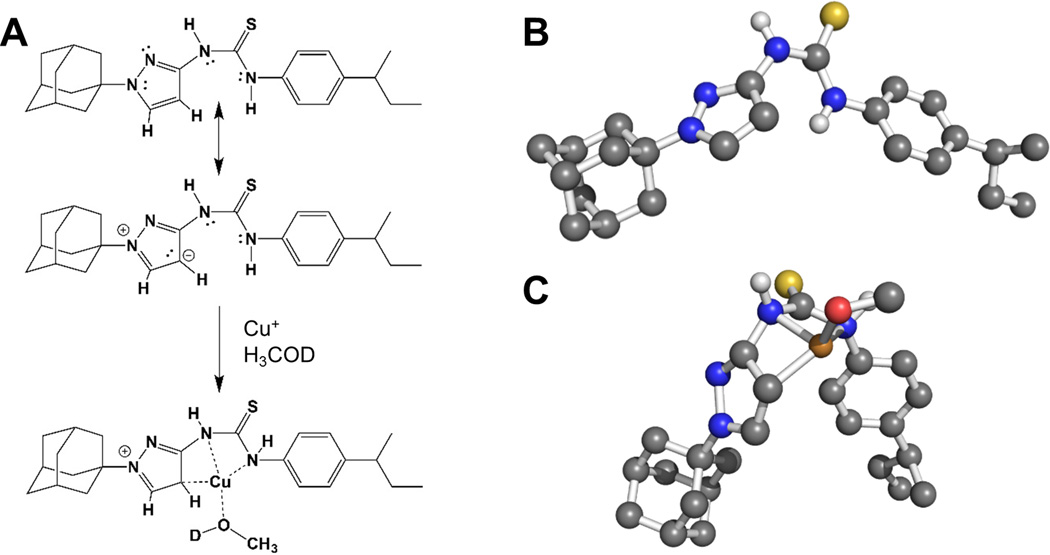
Figure 8. The APT-6i and copper complex has unique coordination chemistry.
The complex’s geometry was determined using UV-Vis and 1H-NMR, shown in Figure 7. (A) The (minor) resonance structure of APT-6i is able to form a complex with Cu(I). (B) 3D representation of uncomplexed APT-6i using the CHARMM force field, showing a relatively linear structure. Non-polar hydrogens are removed for clarity. (C) 3D model of the APT-6i/Cu(I)/CH3OD complex. Coordination twists the molecule from a linear structure to a bent configuration. Copper is represented as the orange sphere, with a D1-methanol added to the coordination complex. Yellow sphere represents sulfur, blue are nitrogen atoms, grey are carbon atoms and white are polar hydrogens.