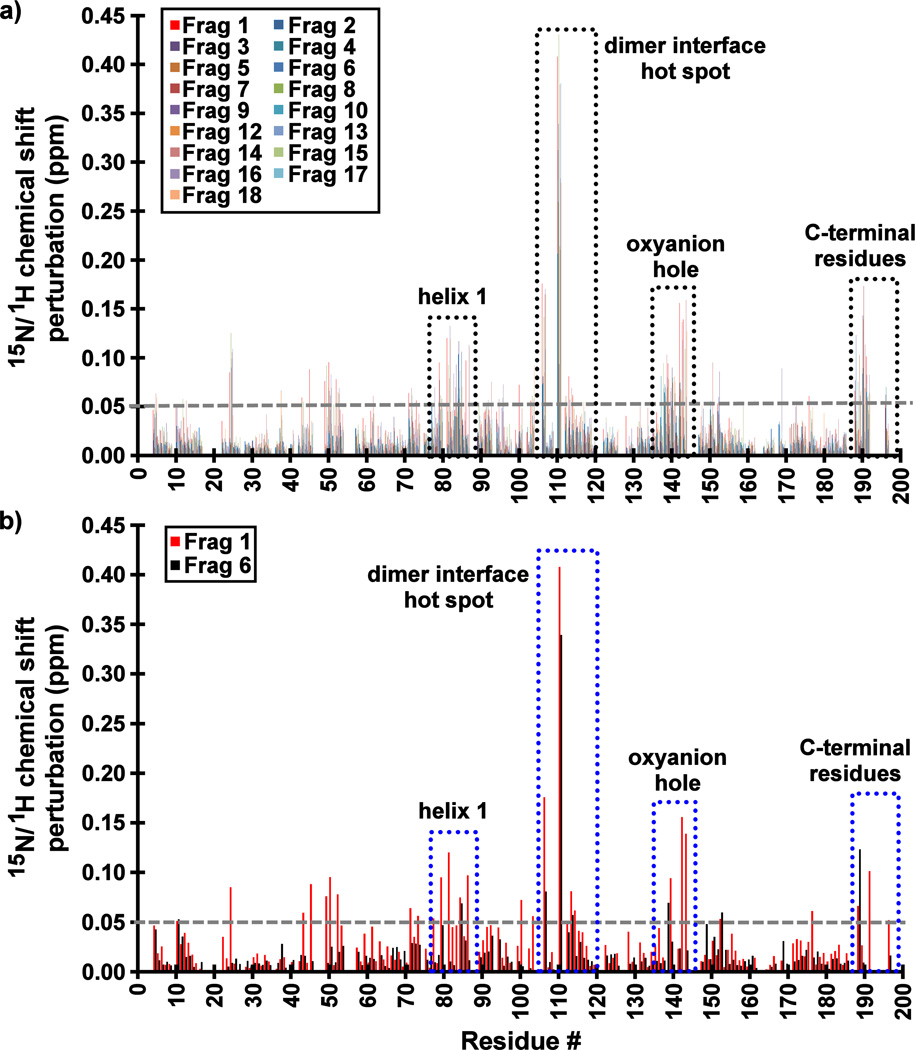
Figure 3.
Backbone 15N/1HN amide chemical shift perturbations (CSPs) of KSHV Pr Δ196 in the presence of 20–25× molar excess of (a) all Table 1 Fragments and (b) Fragments 1 and 6. The most perturbed backbone amides are highlighted in dotted boxes, and include residues at dimer interface near the hot spot W109, the oxyanion hole, helix 1, and the C-terminus. The largest CSP values for those Fragments which demonstrate binding to KSHV Pr are consistently observed for Leu110.