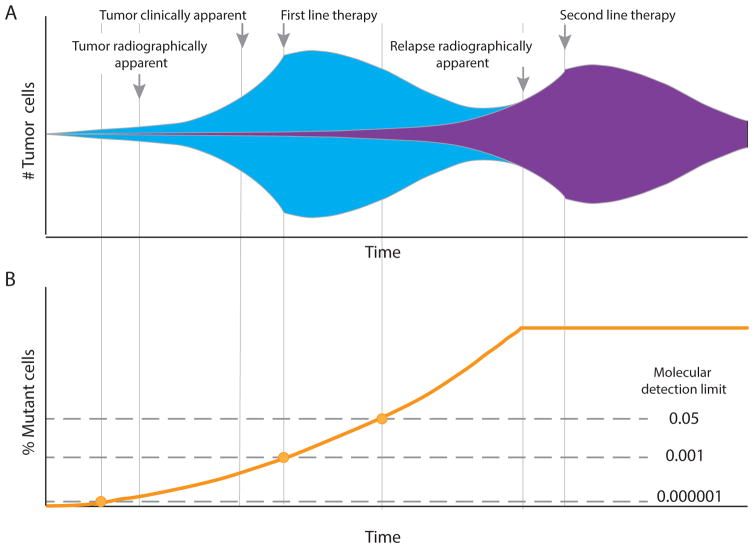
Figure 1.
The evolution and detection of drug-resistance in patients with cancer. a. DNA replication errors can introduce increasing genetic diversity at every cell division after the clonal founding of a tumour; thus, considerable genetic heterogeneity exists in the tumour at the time of diagnosis. As a consequence, a small subset of tumour cells with mutations that confer resistance to particular therapies will often be present (represented by purple shading). Initially, these drug-resistant cells have no specific growth advantage and expand at the same overall rate as the entire tumour; however, with introduction of a therapeutic pressure that hinders the growth of all but the resistant cells, the latter will rapidly takeover the tumour, becoming the predominant clone, until another non-cross-resistant treatment is applied. b. Until recently, a lack of sufficiently sensitive tools to detect these rare subclones meant that resistance could only be identified using clinical criteria, such as radiographic imaging, at a relatively late stage of disease. Newer molecular means for identifying resistance mutations (see ‘Detecting pre-existing drug resistance’ section) are enabling iteratively earlier detection of drug resistance as technical sensitivity improves, and are thus increasing the opportunity to better customize therapy.