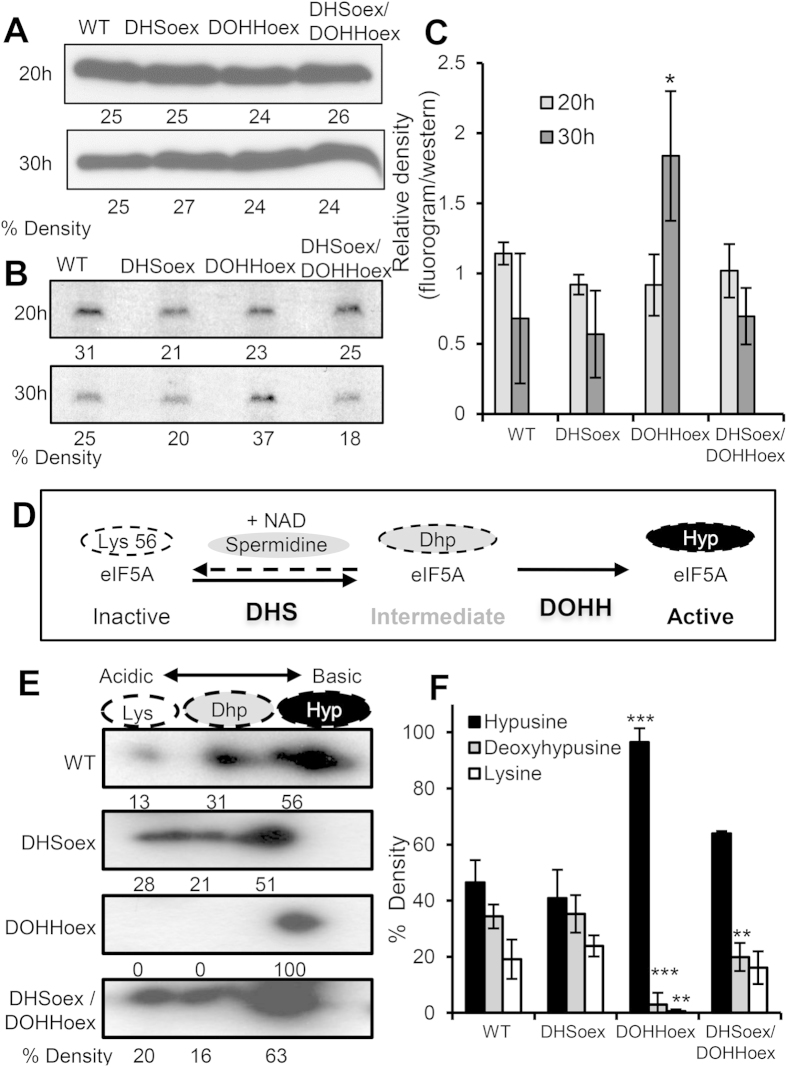
Figure 6. The DOHHoex mutant incorporates more hypusine into eIF5A.
A crude extract of proteins was extracted from mycelia grown for 20 or 30 hours on CM supplemented with 70 μCi of [3H]-spermidine trihydrochloride. (A) Total amounts of eIF5A were determined by Western blotting with an anti-eIF5A antibody, which detected similar amounts of eIF5A protein produced in wild type and overexpressing mutants after 20 h or 30 h of growth in CM. (B) Wild type and overexpressing mutants incorporated similar levels of radiolabelled hypusine after 20 h of growth in CM. However, the DOHHoex mutant incorporated more spermidine after 30 h of growth. (C) The relative un-calibrated density was calculated using ImageJ software. The graph plots the percentage of fluorogram densities (hypusinated eIF5A) divided by the percentage of Western blot densities (total amount of eIF5A). Error bars indicate ± SD calculated from data of three independent experiments. (D) The eIF5A hypusination pathway depicting the DHS bi-directionality and DOHH uni-directionality (modified from 11). (E) The hypusination state of eIF5A in the overexpressing mutants was determined by 2D gel electrophoresis and subsequent Western blots using eIF5A antibody. The three isoforms of eIF5A, inactive (Lys), intermediate deoxyhypusine (Dhp) and active hypusine (Hyp) are detected in the wild type, DHSoex and DHSoex/DOHHoex strains, while the DOHHoex mutant contains only the fully hypusinated (active) form of eIF5A. (F) Percentage un-calibrated density was calculated using ImageJ. Error bars indicate ± SD calculated from data of three independent experiments (n = 3). Significance with respect to wild type: *p < 0.05, **p < 0.01, ***p < 0.001 (calculated with ANOVA-Bonferroni-Holm).