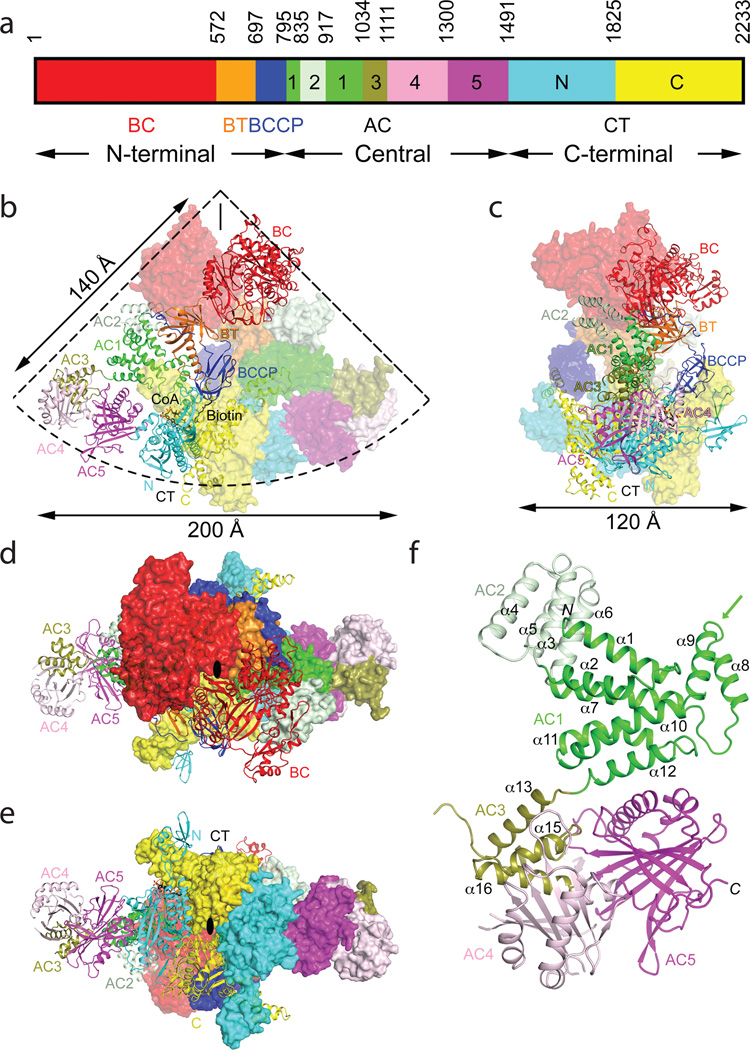
Figure 1.
Crystal structure of the 500 kD yeast acetyl-CoA carboxylase (ScACC) holoenzyme dimer. (a). Domain organization of ScACC. The three regions of the sequence are also indicated. AC: ACC central. (b). Overall structure of ScACC holoenzyme dimer. One protomer is shown as ribbons while the other is shown only as a surface for clarity, both colored according to panel a. The two-fold axis of the dimer is vertical (black line). Overall structure of ScACC holoenzyme, viewed from the side (c), down the BC domain dimer (d), and down the CT domain dimer (e). The two-fold axis is indicated with the black oval. (f). Structure of the five domains (AC1–5) in the central region of ScACC. The arrow points to the helical hairpin insert of AC1. The structure figures were produced with PyMOL (www.pymol.org).