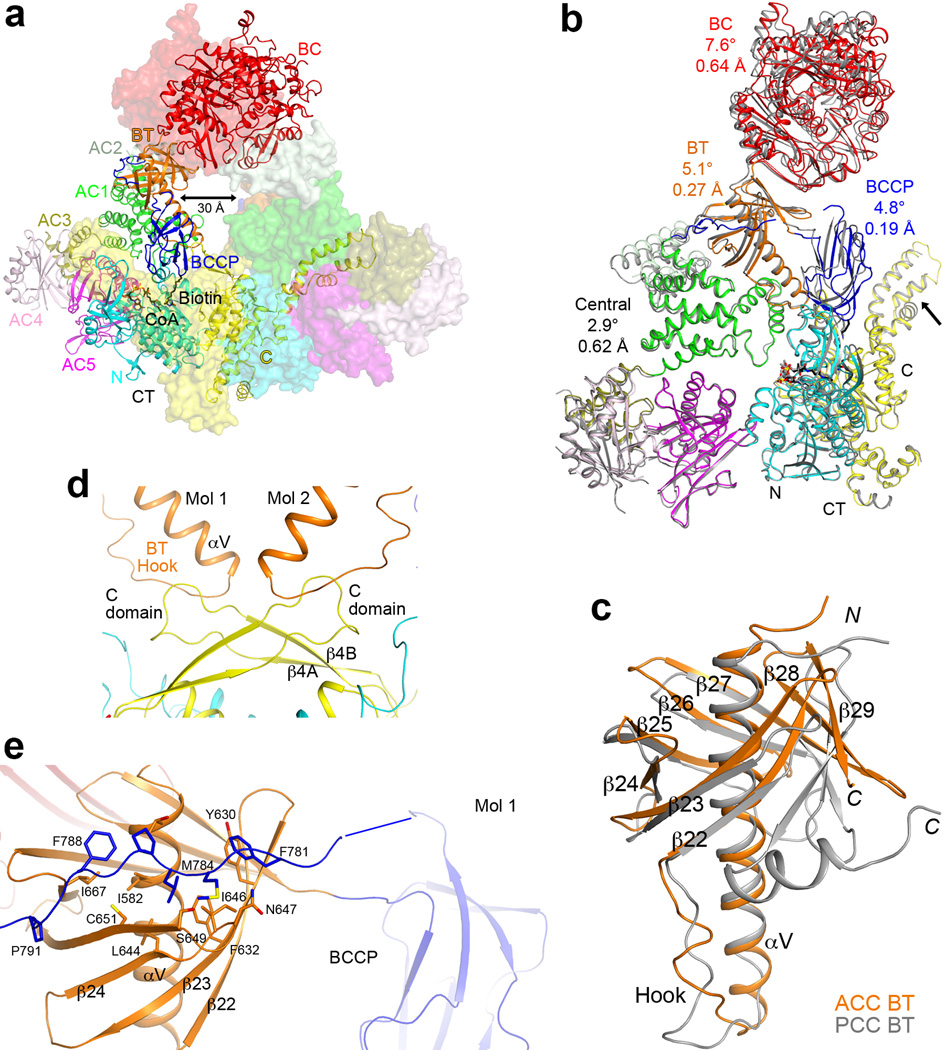
Extended Data Fig. 5.
Overall structure of the ScACC holoenzyme. (a). A large channel in the center of the ScACC holoenzyme dimer. The view is related to that of Fig. 1b by an ~30° rotation around the vertical axis. (b). Overlay of the structures of the two protomers of ScACC holoenzyme dimer. One protomer is shown in color and other in gray. The overlay is based on the CT domain. Differences in the orientations of the other domains are indicated, as well as the rms distance for their equivalent Cα atoms. The arrow points to conformational differences in the insert domain of CT, linked to differences in the BCCP binding mode. (c). Overlay of the BT domain of ScACC (in orange) and the BT domain of PCC (in gray). The ‘hook’, connecting the end of the helix (αV) to the first strand of the β-barrel (β22), is labeled. The last three strands of the β-barrel (β27–β29) are splayed further away from the central helix in ScACC compared to PCC. (d). Interactions between the hook of the BT domain and the β4A–β4B loop from the C domain of CT, an inserted segment that projects away from the rest of the domain. (e). The BCCP-AC1 linker has hydrophobic interactions with the top of one side of the BT domain β-barrel. The disordered segment of the linker is indicated with the blue line.